Abstract
Background
The prognostic impact of positive peritoneal lavage cytology on pancreatic cancer is unclear. Therefore, this study aimed to evaluate its impact in resectable pancreatic body and tail cancer.
Methods
Between January 2006 and December 2019, 97 patients with pancreatic body and tail cancer underwent peritoneal lavage cytology and curative resection at our institution. We analyzed the impact of positive peritoneal lavage cytology on clinicopathological factors and on the prognosis of pancreatic body and tail cancer.
Results
Malignant cells were detected in 14 patients (14.4%) using peritoneal lavage cytology. In these patients, the tumor diameter was significantly larger (p < 0.001) and anterior serosal invasion (p = 0.034), splenic artery invasion (p = 0.013), lympho-vessel invasion (p = 0.025), and perineural invasion (p = 0.008) were significantly more frequent. The R1 resection rate was also significantly higher in patients with positive peritoneal lavage cytology than in negative patients (p = 0.015). Positive peritoneal lavage cytology had a significantly poor impact on overall survival (p = 0.001) and recurrence-free survival (p < 0.001). This cytology was also an independent poor prognostic factor for recurrence (p = 0.022) and was associated with peritoneal dissemination and liver metastasis.
Conclusions
Positive peritoneal lavage cytology is considered to be indicative of more systemic disease in patients with resectable pancreatic body and tail cancer than in patients with negative peritoneal lavage cytology. Early detection of pancreatic cancer before it develops micrometastases is important to improve prognosis, and CY+ patients require more intensive multimodality treatment than standard treatment for resectable pancreatic cancer.
Similar content being viewed by others
Introduction
Pancreatic cancer is currently the 4th leading cause of cancer-related death in the USA. With the number of patients increasing, it is expected to become the second leading cause by 2030 [1, 2]. Despite the development of multidisciplinary treatment for pancreatic cancer, the high rate of recurrence leads to a 5-year survival rate < 20% [3,4,5].
Peritoneal lavage cytology is widely used for diagnosing and staging gastric, ovarian, and endometrial cancers [6,7,8]. However, the prognostic impact of positive lavage cytology (CY +) on pancreatic cancer is controversial, with some reports indicating that it is a poor prognostic factor [9,10,11] and others indicating that it has no effect [12,13,14]. Positive peritoneal lavage cytology is reportedly more common in pancreatic body and tail than in pancreatic head cancer [15]. However, few studies have focused on its prognostic impact in this population because fewer numbers of such patients undergo resection compared to those with pancreatic head cancer [16]. Therefore, this study aimed to evaluate the prognostic impact of positive peritoneal lavage cytology on pancreatic body and tail cancer.
Materials and methods
Patients
We retrospectively reviewed 123 consecutive patients who underwent radical resection for preoperatively resectable pancreatic body and tail cancer between January 2006 and December 2019 at Tokai University Hospital, Japan. Staging was performed based on the Union for International Cancer Control (UICC) tumor-node-metastasis (TNM) classification, 8th edition [17]. Based on this guideline, R0 margins were defined as no tumor cells at any of the resection margins, and R1 as tumor cells being present at the margin without macroscopic residual tumor.
The diagnosis of resectability of pancreatic cancer was based on preoperative imaging findings in accordance with the National Comprehensive Cancer Networks (NCCN) guidelines [18]. Postoperative complications were evaluated using the Clavien–Dindo classification [19].
Neoadjuvant treatment and surgery
For pancreatic body and tail cancer, distal pancreatectomy with regional lymph node dissection was performed. Laparoscopic surgery has been increasingly performed for pancreatic cancer and has been adopted at our department. However, during the study period, essentially all pancreatic cancers were resected by laparotomy. There were no major changes in the surgical technique over the study period. Neoadjuvant chemotherapy for resectable pancreatic cancer has been increasingly adopted. During the study period, the decision on neoadjuvant treatment was made by the attending physician, and upfront surgery was generally performed. Radiotherapy is not administered for resectable pancreatic cancer at our department.
Peritoneal lavage cytology
Peritoneal washing and cytological analysis were performed after laparotomy. Normal saline (100 mL) was introduced into the abdominal cavity and gently agitated; the washing solution was collected from the pouch of Douglas. Smears were prepared from the centrifuged deposit and examined by two experienced pathologists after Papanicolaou and Giemsa staining. If one cancer cell was present, positive peritoneal lavage cytology was diagnosed.
Postoperative follow-up and adjuvant chemotherapy
All patients underwent routine postoperative surveillance. Patients undergoing postoperative adjuvant chemotherapy also underwent tumor marker measurement [carcinoembryonic antigen (CEA) and colorectal carcinoma antigen (CA19-9)] monthly for the first 6 months after surgery, then every 3 months until 3 years after surgery, and every 6 months thereafter. Patients not undergoing postoperative adjuvant chemotherapy underwent tumor marker measurement every 3 months. Chest and abdominal computed tomography was performed every 3 months after surgery for the first 3 years, every 6 months for the following 2 years, and annually thereafter. The choice of postoperative adjuvant chemotherapy depended on the attending physician; before 2016, gemcitabine-based chemotherapy was introduced following the result of the CONKO-001 trial [20], and since the results of the JASPAC01 trial, S-1 therapy has been the mainstay [21].
Statistical analysis
All statistical analyses were performed using SPSS (version 26.0; Chicago, IL). The Chi-square and Mann–Whitney U tests were used to analyze categorical and continuous variables, respectively. Overall survival (OS) and recurrence-free survival (RFS) were analyzed using the Kaplan–Meier method, and statistical significance was evaluated using the log-rank test. Univariate and multivariate Cox proportional hazard regression analyses were conducted to identify the prognostic factors for pancreatic body and tail cancer. Multivariate analyses were performed for variables showing p <0.05 in the univariate analyses. A p-value < 0.05 was considered to be statistically significant. The cut-off values for preoperative CEA and CA19-9 that could predict prognosis were calculated using receiver operating characteristic curves.
Results
After excluding 17 cases wherein peritoneal lavage cytology was not performed, two surgery-related deaths, and seven deaths due to other diseases, 97 cases were included in the final analysis (Fig. 1). Of the 97 patients, 51 were males and 46 were females, with a median age of 69 years (range: 45–84 years). The median observation period was 36 months (range: 5–104 months). The TNM stage 1A, 1B, 2A, 2B, and 3 were present in 21, 24, 8, 30, and 14 patients, respectively. R0 resection was achieved in 80 cases (82.5%). For adjuvant chemotherapy, patients received S-1 (n = 51, 52.6%), gemcitabine (n = 17, 17.5%), and gemcitabine combined with S-1 (n = 10, 10.3%). Adjuvant chemotherapy was completed in 61 cases (69.3%). There were 14 CY + (14.4%) and 83 CY− patients (85.6%). The patients’ baseline characteristics are shown in Table 1.
A comparison of clinicopathological factors in the CY + and CY− groups is shown in Table 2. Preoperative CA19-9 level was significantly higher in the CY + group (p = 0.027). There was a higher rate of blood transfusions in the CY + group (p = 0.013), but no significant differences in operative time, blood loss, complications, or length of hospital stay. Among histopathological factors, tumor diameter was significantly larger in the CY + group (p < 0.001), and anterior serosal invasion (p = 0.034), splenic artery invasion (p = 0.013), lympho-vessel invasion (p = 0.025), and perineural invasion (p = 0.008) were significantly more common. The R1 resection rate was significantly higher in the CY + group (p = 0.015).
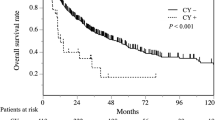
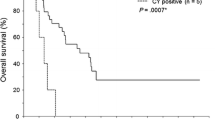
The 1−, 3−, and 5-year survival rates were 64.3%, 17.9%, and 17.9% in the CY + group and 92.8%, 63.5%, and 49.8% in the CY− group, respectively. The median OS of the CY + and CY− groups was 19.0 [95% confidence interval (CI): 2.5–35.5] and 52.0 (95% CI: 31.9–72.1) months, respectively (p = 0.001). The 1−, 3−, and 5-year RFS rates were 28.6%, 7.1%, and 7.1% in the CY + group and 68.7%, 30.3%, and 27.4% in the CY− group, respectively. The median RFS of the CY + and CY− groups was 6.0 (95% CI: 0.6–11.4) and 20.0 (95% CI: 17.1–22.9) months, respectively (p < 0.001). Both OS and RFS were worse in the CY + group (Fig. 2).
When comparing recurrence between the groups, relapse was noted in all the patients in the CY + group, showing a significantly higher rate than that in the CY− group (p = 0.016). In one case in the CY + group, a tumor developed in the residual pancreas after 5 years, and biopsy showed adenocarcinoma. The initial surgery achieved R0 margins. No genetic testing was performed, and whether this was a residual recurrence or an iatrogenic pancreatic cancer was unclear. However, its occurrence does not seem to be directly related to the fact that peritoneal lavage cytology was positive. Liver metastasis and peritoneal dissemination tended to be more common in the CY + group (Table 3). First-line chemotherapy after relapse is shown in Table 4. Gemcitabine plus nab-paclitaxel was administered more frequently in the CY− group (p = 0.007). Five patients (35.7%) in the CY + group and nine (15.0%) in the CY− group received palliative care due to advanced age or poor general condition after relapse (p = 0.085).
In the univariate analysis, the following factors were significantly associated with poor OS: preoperative CEA level (> 4.0 ng/ml), preoperative CA19-9 level (> 65.2 U/ml), positive extrapancreatic plexus invasion, positive venous invasion, positive perineural invasion, CY + , and failure to complete postoperative adjuvant chemotherapy. Multivariate analysis showed that the CA19-9 level (> 65.2 U/ml) [hazard ratio (HR): 2.58, 95% CI: 1.46–4.57, p = 0.001)], positive perineural invasion (HR: 3.38, 95% CI: 1.90–6.02, p < 0.001), UICC-T3, that is tumor diameter > 40 mm (HR: 2.02, 95% CI: 1.09–3.58, p = 0.025), and failure to complete adjuvant chemotherapy (HR: 2.75, 95% CI: 1.54–4.93, p = 0.001) were independent poor prognostic factors. On the other hand, CY + was not significantly associated with OS (Table 5).
Univariate analysis for RFS showed that CEA level (> 4.0 ng/ml), CA19-9 level (> 65.2 U/ml), combined resection of other organs, positive anterior serosal invasion, positive retroperitoneal invasion, positive extrapancreatic plexus invasion, positive splenic artery invasion, positive perineural invasion, CY + status, positive lymph node metastasis, UICC-T3, UICC-stage 3, R1 resection, and failure to complete adjuvant chemotherapy were poor prognostic factors. Multivariate analysis showed that CA19-9 level (> 65.2 U/ml) (HR: 3.91, 95% CI: 2.30–6.66, p < 0.001), positive perineural invasion (HR: 2.43, 95% CI: 1.47–4.02, p = 0.001), UICC-T3 (HR:1.98, 95% CI: 1.03–3.69, p = 0.035), CY + (HR: 2.09, 95% CI: 1.11–3.93, p = 0.022), R1 resection (HR: 2.92, 95% CI: 1.58–5.42, p = 0.001), and failure to complete adjuvant chemotherapy (HR: 3.18, 95% CI: 1.90–5.32, p < 0.001) were poor prognostic factors (Table 6). In terms of RFS, CY + was found to be an independent poor prognostic factor in resectable pancreatic body and tail cancer.
Discussion
In this study, CY + in pancreatic body and tail cancer was significantly associated with poor RFS but not OS. Peritoneal dissemination and liver metastases were significantly more common in the CY + group.
Intraoperative peritoneal lavage cytology is used in the diagnosis of various carcinomas.
CY + has been reported to be a poor prognostic factor in gastric, colorectal and cervical cancers [22,23,24]. However, its prognostic impact depends on the specific cancer, with no effect observed in ovarian and biliary tract cancers [25, 26]. In recent years, CY+ in pancreatic cancer has been considered to have the same status as stage 4 metastatic disease according to the NCCN, UICC and American Joint Committee on Cancer (AJCC) guidelines [17, 18, 27]. Conversely, CY findings are not included in the staging system of the Japan Pancreas Society based on previous studies, and pancreatic cancers are not considered to be unresectable [28].
Pancreatic body and tail cancer has a higher rate of metastasis at the time of diagnosis and poorer prognosis than pancreatic head cancer [29, 30]. While pancreatic head cancer is usually detected subsequent to symptoms such as obstructive jaundice and cholangitis, patients with pancreatic body and tail cancer are less likely to present with symptoms and often have more locally advanced disease at the time of diagnosis. This is one reason why peritoneal washing cytology is often positive in pancreatic body and tail cancer. In the present study, the CY+ group had significantly larger tumors; and more common anterior tissue invasion, lympho-vessel and perineural invasion, and R1 resections, indicating a greater systemic disease burden.
Although the accuracy of diagnostic imaging to determine locally advanced pancreatic cancer has been improving, occult distant metastases that are not detected preoperatively are still observed intraoperatively [31, 32]. In recent years, staging laparoscopy has been performed to determine peritoneal lavage cytology, detect distant metastases, and avoid non-curative surgery [33, 34]. Traverso et al. reported that, among patients diagnosed with unresectable locally advanced pancreatic cancer on preoperative computed tomography, staging laparoscopy found distant metastases in 28% of pancreatic head and 53% of pancreatic body and tail cancers [15]. Moreover, Krabicak et al. reported that staging laparoscopy showed unexpected distant metastases in 46% of pancreatic head and 66% of pancreatic body and tail cancers (p= 0.011). They further reported that pancreatic body and tail cancer diameter ≥ 42 mm is an independent risk factor for peritoneal dissemination, similar to our findings in the CY+ group. They concluded that staging laparoscopy should be performed in patients with large pancreatic body and tail cancer because of the high risk of peritoneal dissemination [35].
The size of pancreatic cancers has been reported to be an important factor in recurrence and prognosis. Although the prognosis worsens with increasing tumor size, smaller tumor still exhibits frequent recurrences and metastases. Ansari et al. reported that distant metastasis occurred in 30.6% of patients with tumors ≤0.5 cm [36]. According to Marchegiani et al., tumors > 20 mm in diameter have a significantly poorer prognosis, with more positive lymph node metastases, poorly differentiated tissue, perineural invasion, and R1 resections, and should be treated with neoadjuvant chemotherapy. They also found that preoperative imaging should be evaluated carefully as it underestimates actual tumor size by nearly 20% [37]. Haeno et al. developed a computational model that predicts metastasis at the time of diagnosis using factors such as primary tumor size. They found that pancreatic cancer growth is initially exponential. This suggests that tumor size at diagnosis is important for predicting metastasis and prognosis. They further stated that it is more important to initiate treatment to control cell proliferation than to perform surgery [38]. Tumor diameter > 40 mm was also independently associated with OS and RFS in this study.
Gemcitabine plus nab-paclitaxel and FOLFIRINOX [a combination of drugs, including: FOL – folinic acid, F – fluorouracil, Irin – irinotecan, and Ox – oxaliplatin] has become the standard treatment for unresectable or recurrent pancreatic cancer. The median OS for these treatments is reported to be 8.5 and 11.1 months, respectively [39, 40]. The median OS in resectable CY+ patients reportedly ranges from 8.0 to 23.8 months [9, 10, 14, 41], a better prognosis than that of patients with pancreatic cancer with distant metastases. On the other hand, locally advanced pancreatic cancer treated with chemotherapy or chemoradiotherapy alone with gemcitabine plus nab-paclitaxel or FOLFIRINOX has shown improved outcomes, with survival ranging from 10.0 to 32.7 months. This prognosis is better than that for CY+ resected cases [42, 43]. Neoadjuvant chemotherapy is being increasingly used in resectable pancreatic cancer [44]. In order to improve the prognosis of CY+ patients, it is necessary to reconsider treatment methods, such as using more potent gemcitabine plus nab-paclitaxel or FOLFIRINOX postoperatively, in addition to neoadjuvant treatment. However, early detection, before the development of micrometastases, remains the most important issue. Recently, liquid biopsy involving tumor cells, circulating tumor DNA, microRNA; and artificial intelligence have been used for early detection of pancreatic cancer [45,46,47].
This study has some limitations. First, this was a retrospective single-center study with a relatively small number of patients. Second, the detection of cancer cells may be underestimated because peritoneal lavage cytology was only performed in the pouch of Douglas. The detection rate is reportedly higher when peritoneal lavage cytology is performed at multiple locations [48]. In addition, many reports use 100–200 ml of saline solution for pancreatic cancer [11,12,13, 16], compared to 10–1000 ml or more for other cancers [49, 50], which may also affect the detection rate. In addition, reverse-transcription polymerase chain reaction reportedly increases detection sensitivity [51]. Finally, the validation in postoperative adjuvant chemotherapy and treatment after recurrence may have led to selection bias. Therefore, a multi-institutional prospective study is needed to clarify the clinical impact and best treatment of CY+ patients with pancreatic body and tail cancer.
In conclusion, positive peritoneal lavage cytology in patients with resectable pancreatic body and tail cancer is considered to be indicative of greater systemic disease. Detection of pancreatic cancer before this occurs remains crucial. Standard treatment for resectable pancreatic cancer does not provide satisfactory prognosis, and these patients require more optimal and intensive multimodality treatment.
References
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68:7–30. https://doi.org/10.3322/caac.21442
Rahib L, Smith BD, Aizenberg R et al (2014) Projection cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 74:2913–2921. https://doi.org/10.1158/0008-5472.CAN-14-0155
Katz MH, Wang H, Fleming JB et al (2009) Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol 16:836–847. https://doi.org/10.1245/s10434-008-0295-2
Vincent A, Herman J, Schulick R, Hruban RH, Goggins M (2011) Pancreatic cancer. Lancet 378:607–620. https://doi.org/10.1016/S0140-6736(10)62307-0
Gupta R, Amanam I, Chung V (2017) Current and future therapies for advanced pancreatic cancer. J Surg Oncol 116:25–34. https://doi.org/10.1002/jso.24623
Jamel S, Markar SR, Malietzis G et al (2018) Prognostic significance of peritoneal lavage cytology in staging gastric cancer: systematic review and meta-analysis. Gastric Cancer 21:10–18. https://doi.org/10.1007/s10120-017-0749-y
Aletti GD, Gallenberg MM, Cliby WA, Jatoi A, Hartmann LC (2007) Current management strategies for ovarian cancer. Mayo Clin Proc 82:751–770. https://doi.org/10.4065/82.6.751
Lee B, Suh DH, Kim K, Kim YB (2016) Influence of positive peritoneal cytology on prognostic factors and survival in early-stage endometrial cancer: a systematic review and meta-analysis. Jpn J Clin Oncol 46:711–717. https://doi.org/10.1093/jjco/hyw063
Ferrone CR, Haas B, Tang L et al (2006) The influence of positive peritoneal cytology on survival in patients with pancreatic adenocarcinoma. J Gastrointest Surg 10:1347–1353. https://doi.org/10.1016/j.gassur.2006.07.013
Hirabayashi K, Imoto A, Yamada M et al (2015) Positive intraoperative peritoneal lavage cytology is a negative prognostic factor in pancreatic ductal adenocarcinoma: A retrospective single-center study. Front Oncol 5:182. https://doi.org/10.3389/fonc.2015.00182
Satoi S, Murakami Y, Motoi F, Uemura K, Kawai M, Kurata M, Sho M, Matsumoto I, Yanagimoto H, Yamamoto T, Mizuma M (2015) Reappraisal of peritoneal washing cytology in 984 patients with pancreatic ductal adenocarcinoma who underwent margin-negative resection. J Gastrointest Surg 19(1):6–14
Yachida S, Fukushima N, Sakamoto M et al (2002) Implications of peritoneal washing cytology in patients with potentially resectable pancreatic cancer. Br J Surg 89:573–578. https://doi.org/10.1046/j.1365-2168.2002.02061.x
Yamada S, Takeda S, Fujii T et al (2007) Clinical implications of peritoneal cytology in potentially resectable pancreatic cancer: positive peritoneal cytology may not confer an adverse prognosis. Ann Surg 246:254–258. https://doi.org/10.1097/01.sla.0000261596.43439.92
Yoshioka R, Saiura A, Koga R et al (2012) The implications of positive peritoneal lavage cytology in potentially resectable pancreatic cancer. World J Surg 36:2187–2191. https://doi.org/10.1007/s00268-012-1622-0
Liu RC, Traverso LW (2005) Diagnostic laparoscopy improves staging of pancreatic cancer deemed locally unresectable by computed tomography. Surg Endosc 19:638–642. https://doi.org/10.1007/s00464-004-8165-x
Iwagami Y, Eguchi H, Wada H et al (2015) Implications of peritoneal lavage cytology in resectable left-sided pancreatic cancer. Surg Today 45:444–450. https://doi.org/10.1007/s00595-014-0964-7
Brierly JD, Gospodarowicz MK, Wittekind C (2016) UICC TMN classification of malignant tumors, 8th edn. Wiley, Hoboken NJ -Blackwell
Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, Chiorean EG, Chung V, Czito B, Del Chiaro M, Dillhoff M (2021) Pancreatic adenocarcinoma, version 2.2021 NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw 19(4):439–457
Clavien PA, Barkun J, de Oliveira ML et al (2009) The Clavien-dindo classification of surgical complications: five-year experience. Ann Surg 250:187–196. https://doi.org/10.1097/SLA.0b013e3181b13ca2
Oettle H, Neuhaus P, Hochhaus A et al (2013) Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 310:1473–1481. https://doi.org/10.1001/jama.2013.279201
Uesaka K, Boku N, Fukutomi A et al (2016) Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 388:248–257. https://doi.org/10.1016/S0140-6736(16)30583-9
Higaki E, Yanagi S, Gotohda N et al (2017) Intraoperative peritoneal lavage cytology offers prognostic significance for gastric cancer patients with curative resection. Cancer Sci 108:978–986. https://doi.org/10.1111/cas.13219
Bosanquet DC, Harris DA, Evans MD, Beynon J (2013) Systematic review and meta-analysis of intraoperative peritoneal lavage for colorectal cancer staging. Br J Surg 100:853–862. https://doi.org/10.1002/bjs.9118
Yoon SH, Kim SN, Shim SH et al (2015) The impact of positive peritoneal cytology on prognosis in patients with cervical cancer: a meta-analysis. Br J Cancer 113:595–602. https://doi.org/10.1038/bjc.2015.266
Douligeris A, Pergialiotis V, Fasoulakis Z, Rodolakis A, Haidopoulos D (2022) Is there a correlation of positive peritoneal washing cytology during interval debulking surgery on the survival outcomes of ovarian cancer patients? A systematic review of ovarian cancer patients? A systematic review and meta-analysis of the literature. J Gynecol Obstet Hum Reprod 51:102256. https://doi.org/10.1016/j.jogoh.2021.102256
Matsukuma S, Nagano H, Kobayashi S, Wada H, Seo S, Tsugawa D, Okuyama H, Iida K, Ohmura Y, Takeda Y, Miyamoto A (2021) The impact of peritoneal lavage cytology in biliary tract cancer (KHBO1701): Kansai Hepato-Biliary Oncology Group. Cancer Rep 4(2):e1323
Amin MB, Edge S, Greene F et al (2017) AJCC Cancer staging system manual, 8th edn. Springer, NY, p 337347
Isaji S, Kawarada Y, Uemoto S (2004) Classification of pancreatic cancer: comparison of Japanese and UICC classifications. Pancreas 28:231–234. https://doi.org/10.1097/00006676-200404000-00003
Sener SF, Fremgen A, Menck HR, Winchester DP (1999) Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the National Cancer Database. J Am Coll Surg 189:1–7. https://doi.org/10.1016/s1072-7515(99)00075-7
Tomasello G, Ghidini M, Costanzo A, Ghidini A, Russo A, Barni S, Passalacqua R, Petrelli F (2019) Outcome of head compared to body and tail pancreatic cancer: A systematic review and meta-analysis of 93 studies. J Gastrointest Oncol 10(2):259
Scialpi M, Reginelli A, D’Andrea A et al (2016) Pancreatic tumors imaging: an update. Int J Surg 28(Supplement 1):S142–S155. https://doi.org/10.1016/j.ijsu.2015.12.053
Cheng H, Luo G, Jin K et al (2021) Predictive values of preoperative markers for resectable pancreatic body and tail cancer determined by MDCT to detect occult metastases. World J Surg 45:2185–2190. https://doi.org/10.1007/s00268-021-06047-x
Yamamura K, Yamashita YI, Yamao T, Kuroda D, Eto T, Kitano Y, Arima K, Miyata T, Okabe H, Nitta H, Hashimoto D (2020) Efficacy of staging laparoscopy for pancreatic cancer. Anticancer Res 40(2):1023–1027
Allen VB, Gurusamy KS, Takwoingi Y, Kalia A, Davidson BR (2016) Diagnostic accuracy of laparoscopy following computed tomography (CT) scanning for assessing the resectability with curative intent in pancreatic and periampullary cancer. Cochrane Database Syst Rev 2016:D9323. https://doi.org/10.1002/14651858.CD009323.pub3
Karabicak I, Satoi S, Yanagimoto H et al (2016) Risk factors for latent distant organ metastasis detected by staging laparoscopy in patients with radiologically defined locally advanced pancreatic ductal adenocarcinoma. J Hepatobiliary Pancreat Sci 23:750–755. https://doi.org/10.1002/jhbp.408
Ansari D, Bauden M, Bergström S et al (2017) Relationship between tumour size and outcome in pancreatic ductal adenocarcinoma. Br J Surg 104:600–607. https://doi.org/10.1002/bjs.10471
Marchegiani G, Andrianello S, Malleo G et al (2017) Does size matter in pancreatic cancer?: Reappraisal of tumour dimension as a predictor of outcome beyond the TNM. Ann Surg 266:142–148. https://doi.org/10.1097/SLA.0000000000001837
Haeno H, Gonen M, Davis MB et al (2012) Computational Modeling of pancreatic cancer reveals kinetics of metastasis suggesting optimum treatment strategies. Cell 148:362–375. https://doi.org/10.1016/j.cell.2011.11.060
Von Hoff DD, Ervin T, Arena FP et al (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369:1691–1703. https://doi.org/10.1056/NEJMoa1304369
Conroy T, Desseigne F, Ychou M et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825. https://doi.org/10.1056/NEJMoa1011923
Cao F, Li J, Li A, Li F (2017) Prognostic significance of positive peritoneal cytology in resectable pancreatic cancer: a systemic review and meta-analysis. Oncotarget 8(9):15004
Suker M, Beumer BR, Sadot E et al (2016) FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol 17:801–810. https://doi.org/10.1016/S1470-2045(16)00172-8
Takada R, Ikezawa K, Daiku K et al (2021) The survival benefit of chemoradiotherapy following induction chemotherapy with gemcitabine plus nab-paclitaxel for unresectable locally advanced pancreatic cancer. Cancers 13:4733. https://doi.org/10.3390/cancers13184733
Motoi F, Unno M (2020) Neoadjuvant treatment for resectable pancreatic adenocarcinoma: what is the best protocol? Ann Gastroenterol Surg 4:100–108. https://doi.org/10.1002/ags3.12311
Yang J, Xu R, Wang C et al (2021) Early screening and diagnosis strategies of pancreatic cancer: a comprehensive review. Cancer Commun Lond 41:1257–1274. https://doi.org/10.1002/cac2.12204
Kenner B, Chari ST, Kelsen D et al (2021) Artificial intelligence and early detection of pancreatic cancer: 2020 summative Review. Pancreas 50:251–279. https://doi.org/10.1097/MPA.0000000000001762
Singhi AD, Koay EJ, Chari ST, Maitra A (2019) Early detection of pancreatic cancer: opportunities and challenges. Gastroenterology 156:2024–2040. https://doi.org/10.1053/j.gastro.2019.01.259
Homma Y, Ushida S, Yamada M, Kobayashi H, Suzuki K (2010) Positive peritoneal washing cytology in multiple cavities can predict poor prognosis of advanced gastric cancer patients. Ann Surg Oncol 17:455–460. https://doi.org/10.1245/s10434-009-0764-2
Junior EJ, das Chagas Medeiros F, (2021) Peritoneal lavage cytology in the diagnosis of pelvic endometriosis. Diagn Cytopathol 49:677–681. https://doi.org/10.1002/dc.24721
Valletti M, Eshmuminov D, Gnecco N et al (2021) Gastric cancer with positive peritoneal cytology: survival benefit after induction chemotherapy and conversion to negative peritoneal cytology. World J Surg Oncol 19:245. https://doi.org/10.1186/s12957-021-02351-x
Eguchi H, Ohigashi H, Takahashi H et al (2009) Presence of minute cancer cell dissemination in peritoneal lavage fluid detected by reverse transcription PCR is an independent prognostic factor in patients with resectable pancreatic cancer. Surgery 146:888–895. https://doi.org/10.1016/j.surg.2009.04.021
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
The study was approved by the Institutional Ethical Board of Tokai University Hospital and was performed in accordance with the Declaration of Helsinki.
Informed consent
The need for informed consent was waived by the Institutional Ethical Board of Tokai University Hospital due to the retrospective nature of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mashiko, T., Ogasawara, T., Masuoka, Y. et al. Prognostic Impact of Positive Peritoneal Lavage Cytology on Resectable Pancreatic Body and Tail Cancer: A Retrospective Study. World J Surg 47, 729–739 (2023). https://doi.org/10.1007/s00268-022-06818-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-022-06818-0