Abstract
Background
Cytoreductive surgery (CRS) with or without hyperthermic intraperitoneal chemotherapy (HIPEC) is a well-recognised treatment option for the management of colorectal peritoneal metastases (CRPM). However, incorporating the routine use of neoadjuvant chemotherapy (NAC) into this management plan is controversial.
Methods
A systematic review and meta-analysis were conducted to evaluate the impact of neoadjuvant chemotherapy on perioperative morbidity and mortality, and long-term survival of patients with CRPM undergoing CRS and HIPEC.
Results
Twelve studies met the inclusion criteria (n = 2,463 patients). Ten were retrospective cohort, one was prospective cohort, and one was a prospective randomised by design. Patients who received NAC followed by CRS and HIPEC experienced no difference in major perioperative morbidity and mortality compared with patients who underwent surgery first (SF). There was no difference in overall survival at 3 years, but at 5 years NAC patients had superior survival (relative risk [RR] 1.31; 95% confidence interval [CI] 1.11–1.54, P < 0.001). There were no differences in 1- and 3-year, disease-free survival (DFS) between groups. Study heterogeneity was generally high across all outcome measures.
Conclusions
Patients who received neoadjuvant chemotherapy did not experience any increase in perioperative morbidity or mortality. The potential improvement in 5-year overall survival in patients receiving NAC is based on limited confidence due to several limitations in the data, but not sufficiently enough to curtail its use. The practice of NAC in this setting will remain heterogeneous and guided by retrospective evidence until prospective, randomised data are reported.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Peritoneal disease confers the worst prognosis amongst all sites of metastatic colorectal cancer.1 The advent of cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) now offers a well-recognised treatment option for up to a quarter of highly selected patients with colorectal peritoneal metastases (CRPM), with 5-year survival rates of 30–40%.2,3,4,5,6
In a recent landmark, randomised trial (PRODIGE 7) where CRS and oxaliplatin-based HIPEC was compared to CRS alone, 83% of both groups received neoadjuvant chemotherapy (NAC).7 The survival data reported for both arms was impressive (41.7 months in the CRS and HIPEC group versus 41.2 months in the CRS group), leading to considerable debate as to the impact of the neoadjuvant, and indeed the adjuvant systemic chemotherapy (AC) in this patient cohort.
The role of neoadjuvant chemotherapy in CRPM is twofold: first to downstage tumour burden while treating micrometastatic systemic disease, but also to aide in patient selection by challenging tumour biology, testing response to chemotherapy, and identifying patients with a favourable disease phenotype.
Current evidence supporting NAC is varied and retrospective in design. Heterogeneity in patient selection, systemic chemotherapy, and intraperitoneal chemotherapy regimens limit interpretation of current data. Two systematic reviews in 2017 highlighted this heterogeneity by not drawing any meaningful conclusions regarding NAC efficacy.8,9 In light of this, a multicentre, randomised trial comparing perioperative chemotherapy (neoadjuvant and adjuvant chemotherapy) CRS and HIPEC versus CRS and HIPEC alone is currently recruiting, with completion predicted for June 2026.10
In the context of new studies since 2017 and the results of PRODIGE 7, this systematic review and meta-analysis aimed to provide a timely, updated assessment of perioperative and oncological outcomes associated with the application of neoadjuvant systemic chemotherapy in patients with CRPM undergoing CRS and HIPEC.
Methods
This study protocol was prospectively registered with PROSPERO (registration number: CRD42021274777) and was performed according to the Preferred Reporting Systems for Systematic Reviews and Meta-Analyses (PRISMA).11
Search strategy
A comprehensive search of the literature was undertaken on Ovid MEDLINE, EMBASE, and the Web of Science databases on the August 12, 2021. The following medical subject heading terms (MESH) were used either alone or in combination, using the explode function: colorectal, peritoneum, CRS, HIPEC, neoadjuvant chemotherapy. The search strategy is supplied as Supplementary Data 1. Search results were pooled using the Rayyan online platform (https://rayyan.qcri.org/welcome).
Inclusion Criteria
Full-text studies with ten or more patients, comparing upfront CRS, HIPEC (surgery first [SF]) to neoadjuvant chemotherapy followed by CRS and HIPEC (NAC) in the treatment of colorectal peritoneal metastases were included. Manual cross-referencing from the bibliographies of papers found in the initial search was undertaken to include additional papers that had not been previously identified. Two reviewers (MF and PW) performed the search and data extraction. The senior authors (AH and MM) independently evaluated any discrepancies in study inclusions or exclusions.
Exclusion Criteria
Case reports or series of ≤10 patients were excluded. Studies reporting on outcomes from appendiceal or noncolorectal cancers were excluded. Similarly, studies reporting on multiple cancers were screened and excluded if data specific to CRPM could not be extracted. Where articles overlapped or duplicated data, those with the more complete or pertinent material were retained. Conference abstracts were not considered.
Data Extraction and Analysis
Data for disease-free (DFS) and overall survival (OS) were collected as percentages at selected intervals of follow-up and converted to absolute numbers for pooled analysis. Median survival data was preferentially not meta-analysed, because two studies did not reach this endpoint.12,13 Overall survival data were extracted without further stratification by subsequent (adjuvant or palliative) systemic chemotherapy or surgical intervention (iterative CRS & HIPEC or palliation). Categorical data, such as perioperative mortality and major (Clavien-Dindo or CTCAE grade III/IV) morbidity, were similarly collected for the final analysis. The assessment of the safety of NAC could not be addressed in this study due to the lack of reported data on chemotherapy-related toxicities and as to whether these toxicities prevented patients in proceeding to surgery. If any data reported zero events, this was replaced with 0.5 to allow for computation of statistical calculation. A pooled odds ratio (OR) and relative risk (RR) was calculated based on the Cochran-Mantel-Haenszel test and random effect model analysis, respectively. I2 statistics was performed to assess for interstudy heterogeneity. P < 0.05 was considered significant. All data analysis was performed in R Studio Team 2015 (RStudio: Integrated Development for R Studio, Inc., Boston, MA), using the metaphor package for meta-analysis.
Risk of Bias Assessment
Two independent reviewers (MF and KW) performed a quality assessment of included studies. This was assessed using the Oxford quality reporting system/Jadad scale for randomised trials14 and the Newcastle Ottawa scale for nonrandomised studies15 (Tables 1 and 2).
Results
Selected Studies
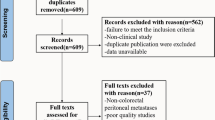
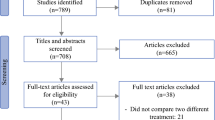
The initial literature search identified 936 articles. After screening, 12 studies were eligible and included in the systematic review and meta-analysis (Fig. 1). Of 2,463 patients included in the 12 studies, 1,273 underwent neoadjuvant systemic chemotherapy followed by CRS and HIPEC (NAC group) and 1,190 underwent upfront CRS and HIPEC (SF group). There was one randomised trial, one prospective cohort, and ten retrospective cohort studies. One study extracted data from the US HIPEC collaborative database.16 Five were single-centre experiences, three were national, bi-institutional, and two were international, multicentre. Ten studies originated from Europe, one from the United States, and one from China.
The characteristics of the 12 included studies are listed in Table 3. The number of patients included who underwent NAC in each study ranged from 14 to 370. Two studies only included patients with synchronous CRPM.12,13 There were a wide range of NAC regimens, with 5-fluorouracil (5-FU), oxaliplatin, and irinotecan used most frequently. The antivascular endothelial growth factor (VEGF) antibody, bevacizumab, was used (where specifically reported) in 41% (196/480) of patients.3,12,13,16,17,18,19
Both the mode and regimen of intraperitoneal chemotherapy varied substantially. Oxaliplatin, mitomycin C, cisplatin, irinotecan, lobaplatin, raltitrexed, and faltitrexed were used in combination or as sole agents as HIPEC. 5-FU was used as early postoperative intraperitoneal chemotherapy (EPIC) in one study.20 Five studies reported on adjuvant systemic chemotherapy by group (NAC versus SF).12,13,16,19,21 Of these, 46% (164/356) of patients who received NAC also received adjuvant chemotherapy compared with 58% (258/444) of patients who underwent surgery first. Six studies reported on mean/median PCI12,13,16,19,22,23 and seven studies reported on the completeness of cytoreduction.12,13,16,19,21,22,23 Operative mean/median PCI ranged from 5 to 15 in the NAC group and 6–14.3 in the SF group. Complete cytoreduction (CC0/1) ranged from 66% to 100% in the NAC group and 46.9–100% in the SF group. Survival data for patients who underwent CRS and HIPEC with or without NAC are listed in Table 4.
Mortality
Seven of the studies (425 NAC/568 SF)12,13,16,19,21,22,23 included in the meta-analysis reported on perioperative mortality. This ranged from 0 to 2.6% in the NAC group, and from 0 to 3% in the SF group. Three studies reported 30-day mortality and four did not specify a timescale. On pooled analysis, patients who received NAC had no associated increased mortality (hazard ratio [HR] 1.05; 95% confidence interval [CI] 0.41-2.65, P = 0.885), with zero heterogeneity (I2 = 0%; Fig. 2).
Morbidity
Six studies (413 NAC/480 SF)12,13,16,19,21,23 reported on major (grade III/IV) perioperative morbidity, which ranged from 22% to 40% in the NAC group and from 16.7% to 33% in the SF group. Four studies reported on major morbidity in accordance with the Clavien-Dindo classification system,13,16,19,23 whereas two reported using Common Terminology Criteria for Adverse Events (CTCAE).12,21 On pooled analysis, there was no difference in grade III/IV morbidity between the two groups (HR 1.08; 95% CI 0.78-1.49; P = 0.719), with zero heterogeneity (I2 = 0%; Fig. 3).
Overall Survival (3-year)
Seven studies (916 NAC/719 SF)13,16,17,18,21,23,24 reported on 3-year overall survival. On pooled analysis, NAC patients experienced no significant improvement in 3-year survival compared with SF patients (RR 1.06; 95% CI 0.94-1.18; P = 0.348), with high heterogeneity (I2 = 74.7%; Fig. 4).
Overall Survival (5-year)
Seven studies (866 NAC/659 SF)3,13,16,18,21,23,24 reported on 5-year overall survival. On pooled analysis NAC patients had a significantly better 5-year overall survival than SF patients (RR 1.31; 95% CI 1.11–1.54; P < 0.001). Heterogeneity was high (I2 = 85.1%; Fig. 5). Given the study by Devilee et al.13 had such a stark difference in survival between groups at 5 years (NAC: 71%, SF: 23%), the pooled analysis was redone without these data. This resulted in a persistent pooled survival advantage for NAC patients (RR 1.22; 95% CI 1.03–1.45; P = 0.024).
Disease-free Survival (1-year)
Disease recurrence was determined by computed tomography ± positron emission tomography in one study,23 by “clinical or radiographic” evidence in another,16 and by unknown means in the third.21 Three studies (306 NAC/307 SF)16,21,23 reported on 1-year disease-free survival. On pooled analysis, NAC patients received no disease-free survival advantage at 1 year compared with SF patients (RR 1.10; 95% CI 0.89–1.35; P = 0.369), with low heterogeneity (I2 = 0%; Fig. 6).
Disease-free Survival (3-year)
Three studies (306 NAC/307 SF)16,21,23 reported on 3-year disease-free survival. On pooled analysis, there was no difference in 3-year, disease-free survival between the groups (RR 0.97; 95% CI 0.89–1.06; P = 0.53), with high heterogeneity (I2 = 76.3%; Fig. 7).
Discussion
This meta-analysis of 12 studies (n = 2,463 patients) demonstrates no significant increase in perioperative major morbidity and mortality in selected patients with CRPM who received preoperative chemotherapy followed by cytoreductive surgery and HIPEC. Despite the predominance of retrospective, low-quality nature of the interpreted data, statistical heterogeneity for both of these outcomes was zero, which allows for relatively confident interpretation. Furthermore, it is worth noting that 41% of patients received bevacizumab as part of their neoadjuvant chemotherapy regimen, further dispelling concerns of its antiangiogenic effects on wound healing and anastomotic integrity25 when ceased appropriately in the preoperative setting. However, interpretation of a survival benefit with NAC is less convincing. No benefit was evident in 1-year and 3-year DFS, although these outcomes were infrequently reported upon across the meta-analysed studies. Examining OS, there was similarly no difference in 3-year survival; however, at 5 years, patients who received NAC had a significant survival advantage.
Supporting rationale for NAC use in patients with “high” PCI/unresectable disease are numerous. The potential of tumour downsizing and elimination of micrometastatic disease increases the likelihood of patients reaching potentially curable surgery, but assessment of factors, such as tumour biology and chemosensitivity, should be considered essential elements to appropriate patient selection. The improvement of 5-year OS in patients with NAC shown in this study may be because of many of these discussion points. The question remains as to whether these aspects hold true for patients with isolated, resectable CRPM.
On subgroup analysis, the PRODIGE 7 trial showed no association between preoperative chemotherapy and overall survival, although interestingly, NAC was deemed a positive prognostic indicator for disease-free survival in the CRS & HIPEC group (HR 0.45; 95% CI 0.25–0.84; P = 0.04). However, data are lacking on the outcomes of patients who were not offered CRS and HIPEC post NAC. It is thus possible that the perceived survival advantage is attributable to patient selection rather than to the chemotherapy itself. The exact benefit, if any, of preoperative systemic chemotherapy may not be decided upon until a prospective, randomised trial data is published. Indeed, in the only randomised trial included in this meta-analysis, Rovers et al., demonstrated the feasibility, safety, and ability of NAC to induce a pathological response.19 This acts as a prelude to the much-awaited survival data that the subsequent phase III trial, CAIRO6, will provide.10
Although not eligible for inclusion here, growing evidence is building on the pathological response of CRPM to NAC. Complementing the complete pathological response (pCR) of 24% shown in the phase II trial by Rovers et al. 19 rates of 11–28% have been described elsewhere,26,27,28 with an accompanying favourable prognosis as is well documented with pCR post neoadjuvant chemoradiotherapy in primary rectal cancer.29 In a prospective study comparing 120 patients with and without a pCR, Bhatt and colleagues26 reported that 80% of patients with a surgical PCI of ≤3 exhibited a pCR, raising further questions as to the benefit of HIPEC in low PCI subgroups. The potential inclination of a surgeon might be to offer CRS & HIPEC upfront to patients with a “low” PCI, but if NAC can induce a pCR without compromising perioperative outcomes, then potentially these patients should be offered initial systemic chemotherapy followed by CRS alone. Interestingly, PRODIGE 7 showed no benefit in HIPEC in patients with a PCI <11 in a heavily pretreated population (83% receiving NAC). Again, this apparent lack of benefit in adding HIPEC may be due to a high pCR rate in this patient subgroup.
The impact of adjuvant chemotherapy was not addressed in this study. It is worth noting, however, that 46% and 58% of the NAC and SF groups (where reported), respectively, received postoperative chemotherapy. The influence of adjuvant systemic chemotherapy on untreated resected CRPM populations has been assessed, albeit retrospectively. A population-based cohort study of almost 400 patients from the Netherlands showed survival benefit in adjuvant chemotherapy (39 months median OS) versus active surveillance (25 months median OS) in patients receiving upfront CRS and HIPEC.30 Similarly from Sweden, a recent retrospective study of 131 consecutive patients reported a median OS of 40 months after complete cytoreduction, with 60% of the study population receiving adjuvant chemotherapy.31 The authors question the need for NAC due to the favourable reported survival. Perhaps, for the purposes of survival, the timing of systemic chemotherapy is inconsequential. However, given the relatively high major morbidity associated with CRS and HIPEC (15–30%)32 and the effect this may have on patients’ ability to receive adjuvant chemotherapy, one could postulate the benefit of giving the total desired amount of systemic chemotherapy in a neoadjuvant fashion, following in the footsteps of total neoadjuvant therapy (TNT) in rectal cancer.33
The largely retrospective design of the included studies leads to an expected high level of selection bias. From experiences at our own centre, further selection bias may lie in those offered upfront surgery. Patients who relapse with resectable peritoneal disease shortly after or even during adjuvant systemic chemotherapy for their index surgery, frequently undergo CRS and HIPEC upfront. Not only have these patients already displayed an aggressive tumour phenotype but also early chemo resistance, possibly precluding any long-term survival.
The lack of reported data on subsequent chemotherapy/surgical intervention (for example redo CRS & HIPEC), which may prolong survival, adds further complexity to the interpretation of the impact of NAC from these studies. This limitation weakens the selection of overall survival as an endpoint in this study.
It is uncertain as to why an overall survival benefit in the neoadjuvant therapy group became significant at 5 years but was not at 3 years. Although not significant at 3 years, the pooled analysis did slightly favour neoadjuvant chemotherapy (RR 1.06). Nevertheless, this discrepancy raises debate as to whether subsequent treatment played a role in survival, even though it appeared that more surgery-first patients received adjuvant systemic chemotherapy, although data on type and number of cycles received was not available. Although one of the studies17 included in the 3-year analysis did not reach adequate follow-up to be included at 5 years, it is unlikely that the 5-year relative risk would change given the trajectory of the Kaplan-Meier curves of each respective subgroup in that study.
Indications for administering systemic chemotherapy rather than upfront surgery were not clear and data surrounding patients not offered surgery post NAC was not reported. Furthermore, diverse differences in both systemic and intraperitoneal chemotherapy regimens (many of which are not widely utilised) add to the heterogeneity of a highly complex and multifaceted disease process.
Conclusions
This systematic review and meta-analysis suggests that neoadjuvant chemotherapy is feasible, and when administered to patients who proceeded to surgery, did not have any direct ill effects on postoperative complications. An overall survival advantage at 5 years was shown in patients who received NAC followed by surgery; however, significant limitations temper confidence in interpretation of this. As we wait for randomised data to provide some clarity on this controversial topic, national and international guidance will continue to be based on low-level evidence.
References
Franko J, Shi Q, Meyers JP, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016;17(12):1709–19.
Razenberg LG, Lemmens VE, Verwaal VJ, et al. Challenging the dogma of colorectal peritoneal metastases as an untreatable condition: Results of a population-based study. Eur J Cancer. 2016;65:113–20.
Baratti D, Kusamura S, Iusco D, et al. Postoperative complications after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy affect long-term outcome of patients with peritoneal metastases from colorectal cancer: a two-center study of 101 patients. Dis Colon Rectum. 2014;57(7):858–68.
Froysnes IS, Larsen SG, Spasojevic M, Dueland S, Flatmark K. Complete cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal peritoneal metastasis in Norway: Prognostic factors and oncologic outcome in a national patient cohort. J Surg Oncol. 2016;114(2):222–7.
Kozman MA, Fisher OM, Rebolledo BJ, et al. CEA to peritoneal carcinomatosis index (PCI) ratio is prognostic in patients with colorectal cancer peritoneal carcinomatosis undergoing cytoreduction surgery and intraperitoneal chemotherapy: A retrospective cohort study. J Surg Oncol. 2018;117(4):725–36.
Maillet M, Glehen O, Lambert J, et al. Early postoperative chemotherapy after complete cytoreduction and hyperthermic intraperitoneal chemotherapy for isolated peritoneal carcinomatosis of colon cancer: a multicenter study. Ann Surg Oncol. 2016;23(3):863–9.
Quenet F, Elias D, Roca L, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):256–66.
Waite K, Youssef H. The role of neoadjuvant and adjuvant systemic chemotherapy with cytoreductive surgery and heated intraperitoneal chemotherapy for colorectal peritoneal metastases: a systematic review. Ann Surg Oncol. 2017;24(3):705–20.
Rovers KP, Simkens GA, Punt CJ, van Dieren S, Tanis PJ, de Hingh IH. Perioperative systemic therapy for resectable colorectal peritoneal metastases: Sufficient evidence for its widespread use? A critical systematic review. Crit Rev Oncol Hematol. 2017;114:53–62.
Rovers KP, Bakkers C, Simkens G, et al. Perioperative systemic therapy and cytoreductive surgery with HIPEC versus upfront cytoreductive surgery with HIPEC alone for isolated resectable colorectal peritoneal metastases: protocol of a multicentre, open-label, parralel-group, phase II-III, randomised, superiority study (CAIRO6). Bmc Cancer. 2019;19.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Zhou S, Jiang Y, Liang J, Pei W, Zhou Z. Neoadjuvant chemotherapy followed by hyperthermic intraperitoneal chemotherapy for patients with colorectal peritoneal metastasis: a retrospective study of its safety and efficacy. World J Surg Oncol. 2021;19(1):151.
Devilee RA, Simkens GA, van Oudheusden TR, et al. Increased survival of patients with synchronous colorectal peritoneal metastases receiving preoperative chemotherapy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2016;23(9):2841–8.
Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12.
GA Wells BS, D O'Connell, J Peterson, V Welch, M Losos, P Tugwell. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.
Beal EW, Suarez-Kelly LP, Kimbrough CW, et al. Impact of neoadjuvant chemotherapy on the outcomes of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal peritoneal metastases: a multi-institutional retrospective review. J Clin Med. 2020;9(3).
Ceelen W, Van Nieuwenhove Y, Vande Putte D, Pattyn P. Neoadjuvant chemotherapy with bevacizumab may improve outcome after cytoreduction and hyperthermic intraperitoneal chemoperfusion (HIPEC) for colorectal carcinomatosis. Annals of Surgical Oncology. 2014;21(9):3023–8.
Passot G, Vaudoyer D, Cotte E, et al. Progression following neoadjuvant systemic chemotherapy may not be a contraindication to a curative approach for colorectal carcinomatosis. Ann Surg. 2012;256(1):125–9.
Rovers KP, Bakkers C, Nienhuijs SW, et al. Perioperative systemic therapy vs cytoreductive surgery and hyperthermic intraperitoneal chemotherapy alone for resectable colorectal peritoneal metastases: a phase 2 randomized clinical trial. JAMA Surg. 2021.
Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22(16):3284–92.
van Eden WJ, Kok NF, Jozwiak K, et al. Timing of systemic chemotherapy in patients with colorectal peritoneal carcinomatosis treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Dis Colon Rectum. 2017;60(5):477–87.
Leimkuhler M, Hemmer PHJ, Reyners AKL, et al. Neoadjuvant chemotherapy followed by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal cancer: a feasibility and safety study. World J Surg Oncol. 2019;17(1):14.
Repullo DJ, Barbois S, Leonard D, et al. The absence of benefit of perioperative chemotherapy in initially resectable peritoneal metastases of colorectal cancer origin treated with complete cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: A retrospective analysis. Eur J Surg Oncol. 2021;47(7):1661–7.
Elias D, Gilly F, Boutitie F, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28(1):63–8.
Eveno C, Passot G, Goere D, et al. Bevacizumab doubles the early postoperative complication rate after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol. 2014;21(6):1792–800.
Bhatt A, Rousset P, Benzerdjeb N, et al. Clinical and radiologic predictors of a pathologic complete response to neoadjuvant chemotherapy (NACT) in patients undergoing cytoreductive surgery for colorectal peritoneal metastases: results of a prospective multi-center study. Ann Surg Oncol. 2021;28(7):3840–9.
Mor E, Assaf D, Laks S, et al. Ratio of pathological response to preoperative chemotherapy in patients undergoing complete cytoreduction and hyperthermic intraperitoneal chemotherapy for metastatic colorectal cancer correlates with survival. Ann Surg Oncol. 2021.
Taibi A, Lo Dico R, Kaci R, Naneix AL, Mathonnet M, Pocard M. Impact of preoperative chemotherapy on the histological response of patients with peritoneal metastases from colorectal cancer according to peritoneal regression grading score (PRGS) and TRG. Surg Oncol. 2020;33:158–63.
Kong JC, Guerra GR, Warrier SK, et al. Prognostic value of tumour regression grade in locally advanced rectal cancer: a systematic review and meta-analysis. Colorectal Dis. 2018;20(7):574–85.
Rovers KP, Bakkers C, van Erning FN, et al. Adjuvant systemic chemotherapy vs active surveillance following up-front resection of isolated synchronous colorectal peritoneal metastases. JAMA Oncol. 2020;6(8):e202701.
Ljunggren M, Nordenvall C, Palmer G. Direct surgery with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for patients with colorectal peritoneal metastases. Eur J Surg Oncol. 2021.
Flood M, Narasimhan V, Waters P, et al. Survival after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal peritoneal metastases: a systematic review and discussion of latest controversies. Surgeon. 2021;19(5):310–20.
Kong JC, Soucisse M, Michael M, et al. Total neoadjuvant therapy in locally advanced rectal cancer: a systematic review and metaanalysis of oncological and operative outcomes. Ann Surg Oncol. 2021;28(12):7476–86.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors have no conflicts of interest. There is no grant support or financial relationship associated with this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Flood, M.P., Kong, J.C.H., Wilson, K. et al. The Impact of Neoadjuvant Chemotherapy on the Surgical Management of Colorectal Peritoneal Metastases: A Systematic Review and Meta-Analysis. Ann Surg Oncol 29, 6619–6631 (2022). https://doi.org/10.1245/s10434-022-11699-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-11699-7