Abstract
Background
Peritoneal metastases (PM) from ovarian, gastric, appendiceal, or colorectal origin can be treated via cytoreductive surgery with or without the addition of hyperthermic intraperitoneal chemotherapy (HIPEC) for selected patients. Unfortunately, not all patients are candidates for aggressive surgical debulking. For these patients, pressurized intraperitoneal aerosolized chemotherapy (PIPAC) has emerged as an alternative method for intraperitoneal (IP) chemotherapy administration. This report presents the design and implementation of the first phase 1 trial to evaluate the safety and efficacy of PIPAC in the United States.
Methods
This is an ongoing prospective phase 1 clinical trial of PIPAC for patients who have histologically confirmed ovarian, uterine, gastric, appendiceal, or colorectal cancer with PM and have progressed to at least one evidence-based chemotherapeutic regimen. The trial has two clinical arms. The patients in arm 1 have gynecologic and gastric malignancies treated with IP cisplatin and doxorubicin, and the arm 2 patients have colorectal and appendiceal malignancies treated with intravenous fluorouracil and leucovorin followed by IP oxaliplatin. All the patients are monitored for dose-limiting toxicities and adverse events.
Results
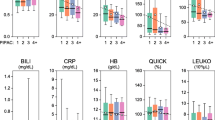
Practical and technical considerations for the phase 1 PIPAC trial are presented. These considerations include patient selection, operating room setup, and technical details for successful aerosolized chemotherapy delivery. The phase 1 study results will be reported separately at completion of the trial.
Conclusions
The PIPAC treatment is a feasible, minimally invasive approach that permits IP delivery of chemotherapy. Once completed, the ongoing phase 1 trial will help to provide safety and initial efficacy data.



Similar content being viewed by others
References
Sugarbaker PH. Peritoneal metastases from gastrointestinal cancer. Curr Oncol Rep. 2018;20:62. https://doi.org/10.1007/s11912-018-0703-0.
Lambert LA. Looking up: recent advances in understanding and treating peritoneal carcinomatosis. CA Cancer J Clin. 2015;65:284–98. https://doi.org/10.3322/caac.21277.
Neuwirth MG, Alexander HR, Karakousis GC. Then and now: cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC), a historical perspective. J Gastrointest Oncol. 2016;7:18–28. https://doi.org/10.3978/j.issn.2078-6891.2015.106.
Sugarbaker PH. Surgical management of peritoneal carcinosis: diagnosis, prevention, and treatment. Langenbecks Arch Chir. 1988;373:189–96. https://doi.org/10.1007/BF01274232.
Long RT, Spratt JS, Dowling E. Pseudomyxoma peritonei: new concepts in management with a report of seventeen patients. Am J Surg. 1969;117:162–9. https://doi.org/10.1016/0002-9610(69)90300-6.
Ghosh BC, Huvos AG, Whiteley HW. Pseudomyxoma peritonei. Dis Colon Rectum. 1972;15:420–5. https://doi.org/10.1007/BF02642643.
Griffiths CT, Parker LM, Fuller AF. Role of cytoreductive surgical treatment in the management of advanced ovarian cancer. Cancer Treat Rep. 1979;63:235–40.
Griffiths CT. Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. Natl Cancer Inst Monogr. 1975;42:101–4.
Munnell EW. Surgical treatment of ovarian carcinoma. Clin Obstet Gynecol. 1969;12:980–92. https://doi.org/10.1097/00003081-196912040-00011.
Zhu Y, Hanna N, Boutros C, Alexander HR. Assessment of clinical benefit and quality of life in patients undergoing cytoreduction and hyperthermic intraperitoneal chemotherapy (HIPEC) for management of peritoneal metastases. J Gastrointest Oncol. 2013;4:62–71. https://doi.org/10.3978/j.issn.2078-6891.2012.053.
Los G, van Vugt MJ, den Engelse L, Pinedo HM. Effects of temperature on the interaction of cisplatin and carboplatin with cellular DNA. Biochem Pharmacol. 1993;46:1229–37. https://doi.org/10.1016/0006-2952(93)90472-9.
Giovanella BC, Stehlin JS, Morgan AC. Selective lethal effect of supranormal temperatures on human neoplastic cells. Cancer Res. 1976;36(11 Pt 1):3944–50.
Hettinga JV, Konings AW, Kampinga HH. Reduction of cellular cisplatin resistance by hyperthermia: a review. Int J Hyperth. 1997;13:439–57.
Shiu MH, Fortner JG. Intraperitoneal hyperthermic treatment of implanted peritoneal cancer in rats. Cancer Res. 1980;40:4081–4.
Meyer JL, Kapp DS, Fessenden P, Hahn GH. Hyperthermic oncology: current biology, physics, and clinical results. Pharmacol Ther. 1989;42:251–88. https://doi.org/10.1016/0163-7258(89)90038-7.
van Driel WJ, Koole SN, Sonke GS. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378:1363–4. https://doi.org/10.1056/NEJMc1802033.
Huo YR, Richards A, Liauw W, Morris DL. Hyperthermic intraperitoneal chemotherapy (HIPEC) and cytoreductive surgery (CRS) in ovarian cancer: a systematic review and meta-analysis. Eur J Surg Oncol. 2015;41:1578–89. https://doi.org/10.1016/j.ejso.2015.08.172.
Hotopp T. HIPEC and CRS in peritoneal metastatic gastric cancer: who really benefits? Surg Oncol. 2019;28:159–66. https://doi.org/10.1016/j.suronc.2019.01.005.
Chua TC, Moran BJ, Sugarbaker PH, et al. Early and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30:2449–56. https://doi.org/10.1200/JCO.2011.39.7166.
Esquivel J, Piso P, Verwaal V, et al. American Society of Peritoneal Surface Malignancies opinion statement on defining expectations from cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with colorectal cancer. J Surg Oncol. 2014;110:777–8. https://doi.org/10.1002/jso.23722.
Bijelic L, Darcy K, Stodghill J, Tian C, Cannon T. Predictors and outcomes of surgery in peritoneal mesothelioma: an analysis of 2000 patients from the National Cancer Database. Ann Surg Oncol. 2020;27:2974–82. https://doi.org/10.1245/s10434-019-08138-5.
Ahmed S, Stewart JH, Shen P, Votanopoulos KI, Levine EA. Outcomes with cytoreductive surgery and HIPEC for peritoneal metastasis. J Surg Oncol. 2014;110:575–84. https://doi.org/10.1002/jso.23749.
Sugarbaker PH. Peritoneal metastases, a frontier for progress. Surg Oncol Clin N Am. 2018;27:413–24. https://doi.org/10.1016/j.soc.2018.02.001.
Spratt JS, Adcock RA, Muskovin M, Sherrill W, McKeown J. Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res. 1980;40:256–60.
Koga S, Hamazoe R, Maeta M, Shimizu N, Kanayama H, Osaki Y. Treatment of implanted peritoneal cancer in rats by continuous hyperthermic peritoneal perfusion in combination with an anticancer drug. Cancer Res. 1984;44:1840–2.
Hamazoe R, Maeta M, Kaibara N. Intraperitoneal thermochemotherapy for prevention of peritoneal recurrence of gastric cancer: final results of a randomized controlled study. Cancer. 1994;73:2048–52. https://doi.org/10.1002/1097-0142(19940415)73:8%3c2048::aid-cncr2820730806%3e3.0.co;2-q.
Fujimura T, Yonemura Y, Muraoka K, et al. Continuous hyperthermic peritoneal perfusion for the prevention of peritoneal recurrence of gastric cancer: randomized controlled study. World J Surg. 1994;18:150–5. https://doi.org/10.1007/BF00348209.
Fujimoto S, Takahashi M, Mutou T, Kobayashi K, Toyosawa T. Successful intraperitoneal hyperthermic chemoperfusion for the prevention of postoperative peritoneal recurrence in patients with advanced gastric carcinoma. Cancer. 1999;85:529–34.
Fujimoto S, Shrestha RD, Kokubun M, et al. Positive results of combined therapy of surgery and intraperitoneal hyperthermic perfusion for far-advanced gastric cancer. Ann Surg. 1990;212:592–6. https://doi.org/10.1097/00000658-199011000-00005.
Girshally R, Demtröder C, Albayrak N, Zieren J, Tempfer C, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) as a neoadjuvant therapy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surg Oncol. 2016;14:253. https://doi.org/10.1186/s12957-016-1008-0.
Solass W, Kerb R, Mürdter T, et al. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol. 2014;21:553–9. https://doi.org/10.1245/s10434-013-3213-1.
Solass W, Kerb R, Mürdter T, et al. PIPAC: pressurized intraperitoneal aerosol chemotherapy: cancer under pressure. Berlin: De Gruyter; 2014. p. 232.
Solass W, Giger-Pabst U, Zieren J, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC): occupational health and safety aspects. Ann Surg Oncol. 2013;20:3504–11. https://doi.org/10.1245/s10434-013-3039-x.
Nadiradze G, Horvath P, Sautkin Y, et al. Overcoming drug resistance by taking advantage of physical principles: pressurized intraperitoneal aerosol chemotherapy (PIPAC). Cancers. 2019. https://doi.org/10.3390/cancers12010034.
Eveno C, Haidara A, Ali I, Pimpie C, Mirshahi M, Pocard M. Experimental pharmacokinetics evaluation of chemotherapy delivery by PIPAC for colon cancer: first evidence for efficacy. Pleura Peritoneum. 2017;2:103–9. https://doi.org/10.1515/pp-2017-0015.
Tempfer CB, Hilal Z, Dogan A, Petersen M, Rezniczek GA. Concentrations of cisplatin and doxorubicin in ascites and peritoneal tumor nodules before and after pressurized intraperitoneal aerosol chemotherapy (PIPAC) in patients with peritoneal metastasis. Eur J Surg Oncol. 2018;44:1112–7. https://doi.org/10.1016/j.ejso.2018.04.020.
Alyami M, Mercier F, Siebert M, et al. Unresectable peritoneal metastasis treated by pressurized intraperitoneal aerosol chemotherapy (PIPAC) leading to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol. 2021;47:128–33. https://doi.org/10.1016/j.ejso.2019.06.028.
Somashekhar SP, Ashwin KR, Rauthan CA, Rohit KC. Randomized control trial comparing quality of life of patients with end-stage peritoneal metastasis treated with pressurized intraperitoneal aerosol chemotherapy (PIPAC) and intravenous chemotherapy. Pleura Peritoneum. 2018;3:20180110. https://doi.org/10.1515/pp-2018-0110.
Odendahl K, Solass W, Demtröder C, et al. Quality of life of patients with end-stage peritoneal metastasis treated with pressurized intraperitoneal aerosol chemotherapy (PIPAC). Eur J Surg Oncol. 2015;41:1379–85. https://doi.org/10.1016/j.ejso.2015.06.001.
Alyami M, Hübner M, Grass F, et al. Pressurised intraperitoneal aerosol chemotherapy: rationale, evidence, and potential indications. Lancet Oncol. 2019;20:e368–77. https://doi.org/10.1016/S1470-2045(19)30318-3.
Tate SJ, Torkington J. Pressurized intraperitoneal aerosol chemotherapy: a review of the introduction of a new surgical technology using the IDEAL framework. BJS Open. 2020;4:206–15. https://doi.org/10.1002/bjs5.50257.
Tempfer CB, Giger-Pabst U, Seebacher V, Petersen M, Dogan A, Rezniczek GA. A phase I, single-arm, open-label, dose escalation study of intraperitoneal cisplatin and doxorubicin in patients with recurrent ovarian cancer and peritoneal carcinomatosis. Gynecol Oncol. 2018;150:23–30. https://doi.org/10.1016/j.ygyno.2018.05.001.
Sundar R, Kim GW, Tan HL, et al. Safety and tolerability of oxaliplatin-based pressurized intraperitoneal aerosol chemotherapy (PIPAC) for patients with peritoneal carcinomatosis: a phase I dose-finding study in Asian patients. J Clin Oncol. 2020;38:360.
Solass W, Sempoux C, Detlefsen S, Carr NJ, Bibeau F. Peritoneal sampling and histological assessment of therapeutic response in peritoneal metastasis: proposal of the Peritoneal Regression Grading Score (PRGS). Pleura Peritoneum. 2016;1:99–107. https://doi.org/10.1515/pp-2016-0011.
Alyami M, Sgarbura O, Khomyakov V, et al. Standardizing training for pressurized intraperitoneal aerosol chemotherapy. Eur J Surg Oncol. 2020;46:2270–5. https://doi.org/10.1016/j.ejso.2020.05.007.
Graversen M, Pedersen PB, Mortensen MB. Environmental safety during the administration of pressurized intraperitoneal aerosol chemotherapy (PIPAC). Pleura Peritoneum. 2016;1:203–8. https://doi.org/10.1515/pp-2016-0019.
Oyais A, Solass W, Zieren J, Reymond MA, Giger-Pabst U. Occupational health aspects of pressurised intraperitoneal aerosol chemotherapy (PIPAC): confirmation of harmlessness. Zentralbl Chir. 2016;141:421–4. https://doi.org/10.1055/s-0033-1350909.
Willaert W, Sessink P, Ceelen W. Occupational safety of pressurized intraperitoneal aerosol chemotherapy (PIPAC). Pleura Peritoneum. 2017;2:121–8. https://doi.org/10.1515/pp-2017-0018.
Ametsbichler P, Böhlandt A, Nowak D, Schierl R. Occupational exposure to cisplatin/oxaliplatin during pressurized intraperitoneal aerosol chemotherapy (PIPAC)? Eur J Surg Oncol. 2018;44:1793–9. https://doi.org/10.1016/j.ejso.2018.05.020.
Delhorme JB, Klipfel A, D’Antonio F, et al. Occupational safety of pressurized intraperitoneal aerosol chemotherapy (PIPAC) in an operating room without laminar airflow. J Visc Surg. 2019;156:485–8. https://doi.org/10.1016/j.jviscsurg.2019.06.010.
Giger-Pabst U, Tempfer CB. How to perform safe and technically optimized pressurized intraperitoneal aerosol chemotherapy (PIPAC): experience after a consecutive series of 1200 procedures. J Gastrointest Surg. 2018;22:2187–93. https://doi.org/10.1007/s11605-018-3916-5.
Graversen M, Detlefsen S, Fristrup C, Pfeiffer P, Mortensen MB. Adjuvant pressurized intraperitoneal aerosol chemotherapy (PIPAC) in resected high-risk colon cancer patients: study protocol for the PIPAC-OPC3 trial: a prospective, controlled phase 2 study. Pleura Peritoneum. 2018;3:20180107. https://doi.org/10.1515/pp-2018-0107.
Schnelle D, Weinreich FJ, Kibat J, Reymond MA. A new ex vivo model for optimizing distribution of therapeutic aerosols: the (inverted) bovine urinary bladder. Pleura Peritoneum. 2017;2:37–41. https://doi.org/10.1515/pp-2017-0006.
Göhler D, Khosrawipour V, Khosrawipour T, et al. Technical description of the microinjection pump (MIP). Surg Endosc. 2017;31:1778–84. https://doi.org/10.1007/s00464-016-5174-5.
Rossi CR, Mocellin S, Pilati P, et al. Pharmacokinetics of intraperitoneal cisplatin and doxorubicin. Surg Oncol Clin N Am. 2003;12:781–94. https://doi.org/10.1016/s1055-3207(03)00030-9.
Andrews PA, Velury S, Mann SC, Howell SB. Cis-diamminedichloroplatinum(II) accumulation in sensitive and resistant human ovarian carcinoma cells. Cancer Res. 1988;48:68–73.
Dumont F, Passot C, Raoul JL, et al. A phase I dose-escalation study of oxaliplatin delivered via a laparoscopic approach using pressurised intraperitoneal aerosol chemotherapy for advanced peritoneal metastases of gastrointestinal tract cancers. Eur J Cancer. 2020;140:37–44. https://doi.org/10.1016/j.ejca.2020.09.010.
Dumont F, Senellart H, Pein F, et al. Phase I/II study of oxaliplatin dose escalation via a laparoscopic approach using pressurized aerosol intraperitoneal chemotherapy (PIPOX trial) for nonresectable peritoneal metastases of digestive cancers (stomach, small bowel, and colorectal): rationale and design. Pleura Peritoneum. 2018;3:20180120. https://doi.org/10.1515/pp-2018-0120.
Gockel I, Jansen-Winkeln B, Haase L, et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) in gastric cancer patients with peritoneal metastasis (PM): results of a single-center experience and Register study. J Gastric Cancer. 2018;18:379–91. https://doi.org/10.5230/jgc.2018.18.e37.
Eveno C, Jouvin I, Pocard M. PIPAC EstoK 01: pressurized intraperitoneal aerosol chemotherapy with cisplatin and doxorubicin (PIPAC C/D) in gastric peritoneal metastasis: a randomized and multicenter phase II study. Pleura Peritoneum. 2018;3:20180116. https://doi.org/10.1515/pp-2018-0116.
Tempfer CB, Winnekendonk G, Solass W, et al. Pressurized intraperitoneal aerosol chemotherapy in women with recurrent ovarian cancer: a phase 2 study. Gynecol Oncol. 2015;137:223–8. https://doi.org/10.1016/j.ygyno.2015.02.009.
Murphy J. Pilot study assessing the efficacy of oxaliplatin-based pressurised intraperitoneal aerosol chemotherapy (PIPAC) for the treatment of colorectal peritoneal metastases. 2021. Retrieved August 10, 2021 from https://clinicaltrials.gov/ct2/show/study/NCT03868228.
Zakharenko A. Oncological benefits of pressured intraperitoneal aerosol chemotherapy (PIPAC) in patients with t3-4 gastric cancer. 2021. Retrieved August 10, 2021 from https://clinicaltrials.gov/ct2/show/study/NCT04595929.
Sgarbura O. Phase II multicenter randomized trial evaluating the association of pipac and systemic chemotherapy versus systemic chemotherapy alone as 1st-line treatment of malignant peritoneal mesothelioma. 2021. Retrieved August 10, 2021 from https://clinicaltrials.gov/ct2/show/study/NCT03875144.
Mortensen M, Fristrup C. ISSPP Launches the new international PIPAC registry. 2021. Retrieved August 10, 2021 from https://isspp.org/isspp-launches-the-new-international-pipac-registry/.
Reymond M, Demtroeder C, Solass W, Winnekendonk G, Tempfer C. Electrostatic precipitation pressurized intraperitoneal aerosol chemotherapy (ePIPAC): first in-human application. Pleura Peritoneum. 2016;1:109–16. https://doi.org/10.1515/pp-2016-0005.
Shariati M, Lollo G, Matha K, et al. Synergy between intraperitoneal aerosolization (PIPAC) and cancer nanomedicine: cisplatin-loaded polyarginine-hyaluronic acid nanocarriers efficiently eradicate peritoneal metastasis of advanced human ovarian cancer. ACS Appl Mater Interfaces. 2020;12:29024–36. https://doi.org/10.1021/acsami.0c05554.
Shariati M, Willaert W, Ceelen W, De Smedt SC, Remaut K. Aerosolization of nanotherapeutics as a newly emerging treatment regimen for peritoneal carcinomatosis. Cancers. 2019. https://doi.org/10.3390/cancers11070906.
Minnaert AK, Dakwar GR, Benito JM, et al. High-pressure nebulization as application route for the peritoneal administration of siRNA COMPLEXES. Macromol Biosci. 2017. https://doi.org/10.1002/mabi.201700024.
Acknowledgments
The authors thank Nicola Welch, PhD, CMPP, for assistance editing the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosure
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Gautam Malhotra and Adrian Kohut equal second co-authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Raoof, M., Malhotra, G., Kohut, A. et al. PIPAC for the Treatment of Gynecologic and Gastrointestinal Peritoneal Metastases: Technical and Logistic Considerations of a Phase 1 Trial. Ann Surg Oncol 29, 175–185 (2022). https://doi.org/10.1245/s10434-021-10505-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-10505-0