Abstract
Background
Although multidisciplinary treatments including the use of adjuvant therapy (AT) have been adopted for biliary tract cancers, patients with distal cholangiocarcinoma (DCC) can still experience recurrence. We sought to characterize the incidence and predictors of early recurrence (ER) that occurred within 12 months following surgery for DCC.
Patients and Methods
Patients who underwent resection for DCC between 2000 and 2015 were identified from the US multi-institutional database. Cox regression analysis was used to identify clinicopathological factors to develop an ER risk score, and the predictive model was validated in an external dataset.
Results
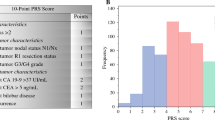
Among 245 patients included in the analysis, 67 patients (27.3%) developed ER. No difference was noted in ER rates between patients who did and did not receive AT (28.7% vs. 25.0%, p = 0.55). Multivariable analysis revealed that neutrophil-to-lymphocyte ratio (NLR), peak total bilirubin (T-Bil), major vascular resection (MVR), lymphovascular invasion, and R1 surgical margin status were associated with a higher ER risk. A DIstal Cholangiocarcinoma Early Recurrence Score was developed according to each factor available prior to surgery [NLR > 9.0 (2 points); peak T-bil > 1.5 mg/dL (1 points); MVR (2 points)]. Cumulative ER rates incrementally increased among patients who were low (0 points; 10.6%), intermediate (1–2 points; 26.8%), or high (3–5 points; 57.6%) risk (p < 0.001) in the training dataset, as well as in the validation dataset [low (0 points); 3.4%, intermediate (1–2 points); 32.7%, or high risk (3–5 points); 55.6% (p < 0.001)].
Conclusions
Among patients undergoing resection for DCC, 1 in 4 patients experienced an ER. Alternative treatment strategies such as neoadjuvant chemotherapy may be considered especially among individuals deemed to be at high risk for ER.



Similar content being viewed by others
References
Bridgewater JA, Goodman KA, Kalyan A, Mulcahy MF. Biliary tract cancer: epidemiology, radiotherapy, and molecular profiling. ASCO. 2016;36:e194–203.
Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–9.
Lopez-Aguiar AG, Ethun CG, Pawlik TM, et al. Association of Perioperative transfusion with recurrence and survival after resection of distal cholangiocarcinoma: a 10-institution study from the US extrahepatic biliary malignancy consortium. Ann Surg Oncol. 2019;26(6):1814–23.
Hatzaras I, George N, Muscarella P, et al. Predictors of survival in periampullary cancers following pancreaticoduodenectomy. Ann Surg Oncol. 2010;17(4):991–7.
Edeline J, Benabdelghani M, Bertaut A, et al. Gemcitabine and Oxaliplatin chemotherapy or surveillance in resected biliary tract cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): a randomized phase III study. J Clin Oncol. 2019;37(8):658–67.
Sahara K, Farooq SA, Tsilimigras DI, et al. Immunotherapy utilization for hepatobiliary cancer in the United States: disparities among patients with different socioeconomic status. Hepatobiliary Surg Nutr. 2020;9(1):13–24.
Zhou W, Qian L, Rong Y, et al. Prognostic factors and patterns of recurrence after curative resection for patients with distal cholangiocarcinoma. Radiother Oncol. 2020;147:111–7.
Courtin-Tanguy L, Turrini O, Bergeat D, et al. Multicentre study of the impact of factors that may affect long-term survival following pancreaticoduodenectomy for distal cholangiocarcinoma. HPB. 2018;20(5):405–10.
Komaya K, Ebata T, Shirai K, et al. Recurrence after resection with curative intent for distal cholangiocarcinoma. Br J Surg. 2017;104(4):426–33.
Nakanishi Y, Okamura K, Tsuchikawa T, et al. Time to Recurrence After surgical resection and survival after recurrence among patients with perihilar and distal cholangiocarcinomas. Ann Surg Oncol. 2020.
Primrose JN, Fox RP, Palmer DH, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019.
Tsilimigras DI, Sahara K, Wu L, et al. Very Early Recurrence after liver resection for intrahepatic cholangiocarcinoma: considering alternative treatment approaches. JAMA Surg. 2020.
Sahara K, Tsilimigras DI, Kikuchi Y, et al. Defining and predicting early recurrence after resection for gallbladder Cancer. Ann Surgical Oncol. 2020.
Lee KJ, Carlin JB. Multiple Imputation for missing data: fully conditional specification versus multivariate normal imputation. Am J Epidemiol. 2010;171(5):624–32.
Groot VP, Gemenetzis G, Blair AB, et al. Defining and predicting early recurrence in 957 patients with resected pancreatic ductal adenocarcinoma. Ann Surg. 2018.
Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–9.
Facciabene A, Peng X, Hagemann IS, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475(7355):226–30.
Tsilimigras DI, Mehta R, Paredes AZ, et al. Overall tumor burden dictates outcomes for patients undergoing resection of multinodular hepatocellular carcinoma beyond the Milan criteria. Ann Surg. 2020;272(4):574–81.
Chan AWH, Zhong J, Berhane S, et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol. 2018;69(6):1284–93.
Sahara K, Tsilimigras DI, Pawlik TM. ASO Author Reflections: Validated prediction model of early recurrence after resection for gallbladder cancer: identifying a subset of patients who may be better served with neoadjuvant therapy. An Surg Oncol. 2020.
Xu XF, Xing H, Han J, et al. Risk factors, patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: a multicenter study from China. JAMA Surg. 2018.
Zhang XF, Beal EW, Bagante F, et al. Early versus late recurrence of intrahepatic cholangiocarcinoma after resection with curative intent. Br J Surg. 2018;105(7):848–56.
Mazzaferro V, Sposito C, Zhou J, et al. Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterol. 2018;154(1):128-139.
Sahara K, Paredes AZ, Tsilimigras DI, et al. Machine learning predicts unpredicted deaths with high accuracy following hepatopancreatic surgery. Hepatobiliary Surg Nutr. 2019.
Semenkovich TR, Yan Y, Subramanian M, et al. A clinical nomogram for predicting node-positive disease in esophageal cancer. Ann Surg. 2019.
Sahara K, Tsilimigras DI, Mehta R, et al. A novel online prognostic tool to predict long-term survival after liver resection for intrahepatic cholangiocarcinoma: The “metro-ticket” paradigm. J Surg Oncol. 2019;120(2):223–30.
Smith RA, Ghaneh P, Sutton R, et al. Prognosis of resected ampullary adenocarcinoma by preoperative serum CA19-9 levels and platelet-lymphocyte ratio. J Gastrointest Surg. 2008;12(8):1422–8.
Moro A, Mehta R, Sahara K, et al. The impact of preoperative CA19-9 and CEA on outcomes of patients with intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2020.
Halazun KJ, Aldoori A, Malik HZ, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur J Surg Oncol (EJSO). 2008;34(1):55–60.
Tsilimigras DI, Moris D, Mehta R, et al. The systemic immune-inflammation index predicts prognosis in intrahepatic cholangiocarcinoma: an international multi-institutional analysis. HPB. 2020.
Tang H, Lu W, Li B, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in biliary tract cancers: a systematic review and meta-analysis. Oncotarget. 2017;8(22):36857–68.
Halazun KJ, Hardy MA, Rana AA, et al. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg. 2009;250(1):141–51.
Zer A, Sung MR, Walia P, et al. Correlation of neutrophil to lymphocyte ratio and absolute neutrophil count with outcomes with PD-1 axis inhibitors in patients with advanced non–small-cell lung cancer. Clin Lung Cancer. 2018;19(5):426-434.e421.
Větvička V, Šíma P, Miler I, Bilej M. The immunosuppressive effects of bilirubin. Folia Microbiol. 1991;36(2):112–9.
Farhat MH, Shamseddine AI, Tawil AN, et al. Prognostic factors in patients with advanced cholangiocarcinoma: role of surgery, chemotherapy and body mass index. W J Gastroenterol. 2008;14(20):3224.
Tempero MA, Behrman S, Ben-Josef E, et al. Pancreatic adenocarcinoma: clinical practice guidelines in oncology. JNCCN. 2005;3(5):598–626.
Katz MHG, Pisters PWT, Evans DB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206(5):833–46.
Matsuyama R, Morioka D, Mori R, et al. Our rationale of initiating neoadjuvant chemotherapy for hilar cholangiocarcinoma: a proposal of criteria for “borderline resectable” in the field of surgery for hilar cholangiocarcinoma. World J Surg. 2018.
Chaudhari VA, Ostwal V, Patkar S, et al. Outcome of neoadjuvant chemotherapy in “locally advanced/borderline resectable” gallbladder cancer: the need to define indications. HPB. 2018;20(9):841–7.
Beane JD, Borrebach JD, Zureikat AH, et al. Optimal pancreatic surgery: are we making progress in North America? Ann Surg. 2019.
Mierke F, Hempel S, Distler M, et al. Impact of portal vein involvement from pancreatic cancer on metastatic pattern after surgical resection. Ann Surg Oncol. 2016;23(5):730–6.
Network NCC. NCCN Clinical Practice Guidelines iin Oncology, Hepatobiliary Cancers. 2020; https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf.
Yadav S, Xie H, Bin-Riaz I, et al. Neoadjuvant versus adjuvant chemotherapy for cholangiocarcinoma: A propensity score matched analysis. Eur J Surg Oncol. 2019;45(8):1432–8.
Goetze TO, Bechstein WO, Bankstahl US, et al. Neoadjuvant chemotherapy with gemcitabine plus cisplatin followed by radical liver resection versus immediate radical liver resection alone with or without adjuvant chemotherapy in incidentally detected gallbladder carcinoma after simple cholecystectomy or in front of radical resection of BTC (ICC/ECC) – a phase III study of the German registry of incidental gallbladder carcinoma platform (GR)– the AIO/ CALGP/ ACO- GAIN-trial –. BMC Cancer. 2020;20(1):122.
Okamura Y, Sugiura T, Ito T, et al. Neutrophil to lymphocyte ratio as an indicator of the malignant behaviour of hepatocellular carcinoma. Br J Surg. 2016;103(7):891–8.
Funding
There was no financial support for this study
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10434_2021_9811_MOESM2_ESM.tiff
Supplementary Fig. 1 KM curves demonstrating differences in OS after recurrence among patients with ER versus LR (TIFF 145 KB)
10434_2021_9811_MOESM3_ESM.tiff
Supplementary Fig. 2 KM curves demonstrating differences in RFS stratified by the DICER score only among patients (a) with and (b) without AT (TIFF 156 KB)
Rights and permissions
About this article
Cite this article
Sahara, K., Tsilimigras, D.I., Toyoda, J. et al. Defining the Risk of Early Recurrence Following Curative-Intent Resection for Distal Cholangiocarcinoma. Ann Surg Oncol 28, 4205–4213 (2021). https://doi.org/10.1245/s10434-021-09811-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-09811-4