Abstract
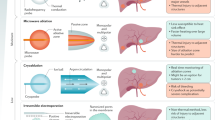
Hepatocellular carcinoma (HCC), the most common primary hepatic malignancy worldwide, is the second leading cause of cancer-related death. Underlying liver dysfunction and advanced stage of disease require treatments to be optimally timed and implemented to minimize hepatic parenchymal damage while maximizing disease response and quality of life. Locoregional therapies (LRTs) such as trans-arterial chemo- and radio-embolization remain effective for intermediate liver-only and advanced HCC disease (i.e., Barcelona-Clinic liver cancer stages B and C) not amendable to primary resection or ablation. Additionally, these minimally invasive interventions have been shown to augment the immune system. This and the recent success of immune-oncologic treatments for HCC have generated interest in applying these therapies in combination with such locoregional interventions to improve patient outcomes and response rates. This report reviews the use of trans-arterial LRTs with immunotherapy for stages B and C HCC, potential biomarkers, and imaging methods for assessing the response and safety of such combinations.
Similar content being viewed by others
References
Clark T, et al. Hepatocellular carcinoma: review of epidemiology, screening, imaging diagnosis, response assessment, and treatment. Curr Probl Diagn Radiol. 2015;44:479–86.
Sim HW, Knox J. Hepatocellular carcinoma in the era of immunotherapy. Curr Probl Cancer. 2018;42:40–8.
Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1.
Fujiwara N, et al. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol. 2018;68:526–49.
Hage C, et al. Characterizing responsive and refractory orthotopic mouse models of hepatocellular carcinoma in cancer immunotherapy. PLoS ONE. 2019;14:e0219517.
Gbolahan OB, et al. Locoregional and systemic therapy for hepatocellular carcinoma. J Gastrointest Oncol. 2017;8:215–28.
Gao Q, et al. Heterogeneity of intermediate-stage HCC necessitates personalized management including surgery. Nat Rev Clin Oncol. 2015;12:10.
Bteich F, Di Bisceglie AM. Current and future systemic therapies for hepatocellular carcinoma. Gastroenterol Hepatol. 2019;15:266–72.
Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–14.
Kim JM, et al. Patients with unresectable hepatocellular carcinoma beyond milan criteria: should we perform transarterial chemoembolization or liver transplantation? Transplant Proceed. 2010;42:821–4.
Jianyong L, et al. Barcelona Clinic liver cancer stage B hepatocellular carcinoma: transarterial chemoembolization or hepatic resection? Medicine. 2014;93:e180.
Greten TF, et al. Combined locoregional-immunotherapy for liver cancer. J Hepatol. 2019;70:999–1007.
Greten TF, Duffy AG, Korangy F. Hepatocellular carcinoma from an immunologic perspective. Clin Cancer Res. 2013;19:6678–85.
Wissniowski TT, et al. Activation of tumor-specific T lymphocytes by radio-frequency ablation of the VX2 hepatoma in rabbits. Cancer Res. 2003;63:6496–500.
Kudo M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69:1492.
Lencioni R, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J Hepatol. 2016;64:1090–8.
Slovak R, et al. Immuno-thermal ablations: boosting the anticancer immune response. J Immunother Cancer. 2017;5:78.
Dendy MS, et al. Locoregional therapy, immunotherapy, and the combination in hepatocellular carcinoma: future directions. Liver Cancer. 2019;8:326–40.
El-Khoueiry AB, et al. Phase I/II safety and antitumor activity of nivolumab in patients with advanced hepatocellular carcinoma (HCC): CA209-040. J Clin Oncol. 2015;33(18 Suppl):LBA101.
Sangro B, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81–8.
Zhu AX, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–52.
Finn RS, et al. Results of KEYNOTE-240: phase 3 study of pembrolizumab (Pembro) vs best supportive care (BSC) for second-line therapy in advanced hepatocellular carcinoma (HCC). J Clin Oncol. 2019;37(15 Suppl):4004.
El-Khoueiry AB, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–502.
Yau T, et al. Nivolumab (NIVO) + ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): results from CheckMate 040. J Clin Oncol. 2019;37(15 Suppl):4012.
Yau T, et al. CheckMate 459: a randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2019;30:v874–5.
Hiroishi K, et al. Strong CD8 + T-cell responses against tumor-associated antigens prolong the recurrence-free interval after tumor treatment in patients with hepatocellular carcinoma. J Gastroenterol. 2010;45:451–8.
Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12:681–700.
Gamrekelashvili J, Greten TF, Korangy F. Immunogenicity of necrotic cell death. CMLS Cell Mol Life Sci. 2015;72:273–83.
Scheffer SR, et al. Apoptotic, but not necrotic, tumor cell vaccines induce a potent immune response in vivo. Int J Cancer. 2003;103:205–11.
Gamrekelashvili J, et al. Peptidases released by necrotic cells control CD8 + T cell cross-priming. J Clin Invest. 2013;123:4755–68.
Ayaru L, et al. Unmasking of alpha-fetoprotein-specific CD4(+) T cell responses in hepatocellular carcinoma patients undergoing embolization. J Immunol. 2007;178:1914–22.
den Brok MH, et al. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br J Cancer. 2006;95:896–905.
Duffy AG, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545–51.
Brar G, Greten TF, Brown ZJ. Current frontline approaches in the management of hepatocellular carcinoma: the evolving role of immunotherapy. Ther Adv Gastroenterol. 2018;11:1756284818808086.
Qin S, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21:571–80.
Wainberg ZA, et al. Safety and clinical activity of durvalumab monotherapy in patients with hepatocellular carcinoma (HCC). J Clin Oncol. 2017;35(15 Suppl):4071.
Finn RS, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–905.
Raoul JL, et al. Updated use of TACE for hepatocellular carcinoma treatment: how and when to use it based on clinical evidence. Cancer Treat Rev. 2019;72:28–36.
Golfieri R, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111:255–64.
Lammer J, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent. Radiol. 2010;33:41–52.
Malagari K, et al. Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with BeadBlock for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2010;33:541–51.
Boulin M, et al. Transarterial chemoembolization for hepatocellular carcinoma: an old method, now flavor of the day. Diagn Intervent Imaging. 2015;96:607–15.
Marelli L, et al. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol. 2007;30:6–25.
Apetoh L, et al. Immunogenicity of anthracyclines: moving towards more personalized medicine. Trends Mol Med. 2008;14:141–51.
Shin SW. The current practice of transarterial chemoembolization for the treatment of hepatocellular carcinoma. Korean J Radiol. 2009;10:425–34.
Favelier S, et al. Lipiodol trans-arterial chemoembolization of hepatocellular carcinoma with idarubicin: first experience. Cardiovasc Intervent Radiol. 2013;36:1039–46.
Rios-Doria J, et al. Doxil synergizes with cancer immunotherapies to enhance antitumor responses in syngeneic mouse models. Neoplasia New York. 2015;17:661–70.
Wang Y-J, et al. Immunogenic effects of chemotherapy-induced tumor cell death. Genes Dis. 2018;5:194–203.
Akinwande O, et al. Is radioembolization ([90]Y) better than doxorubicin drug-eluting beads (DEBDOX) for hepatocellular carcinoma with portal vein thrombosis? A retrospective analysis. Surg Oncol. 2015;24:270–5.
Lencioni R, et al. Transcatheter treatment of hepatocellular carcinoma with doxorubicin-loaded DC bead (DEBDOX): technical recommendations. Cardiovasc Intervent Radiol. 2012;35:980–5.
Martin R, et al. Optimal technique and response of doxorubicin beads in hepatocellular cancer: bead size and dose. Korean J Hepatol. 2011;17:51–60.
Martin RC II, et al. Hepatic arterial infusion of doxorubicin-loaded microsphere for treatment of hepatocellular cancer: a multi-institutional registry. J Am Coll Surg. 2011;213:493–500.
Ding M, et al. Is adjuvant cellular immunotherapy essential after TACE-predominant minimally-invasive treatment for hepatocellular carcinoma? A systematic meta-analysis of studies including 1774 patients. PloS ONE. 2016;11:e0168798.
Ma Y, et al. Cytokine-induced killer (CIK) cell therapy for patients with hepatocellular carcinoma: efficacy and safety. Exp Hematol Oncol. 2012;1:11.
Huang ZM, et al. Cytokine-induced killer cells in combination with transcatheter arterial chemoembolization and radiofrequency ablation for hepatocellular carcinoma patients. J Immunother. 2013;36:287–93.
Duffy AG, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;6:545–51.
Bhangoo MS, et al. Radioembolization with yttrium-90 microspheres for patients with unresectable hepatocellular carcinoma. J Gastrointest Oncol. 2015;6:469–78.
Sato K, et al. Treatment of unresectable primary and metastatic liver cancer with yttrium-90 microspheres (TheraSphere): assessment of hepatic arterial embolization. Cardiovasc Intervent Radiol. 2006;29:522–9.
Golden EB, et al. The convergence of radiation and immunogenic cell death signaling pathways. Front Oncol. 2012;2:88.
Nehs MA, et al. Necroptosis is a novel mechanism of radiation-induced cell death in anaplastic thyroid and adrenocortical cancers. Surgery. 2011;150:1032–9.
Ma Y, et al. Chemotherapy and radiotherapy: cryptic anticancer vaccines. Semin Immunol. 2010;22:113–24.
Demaria S, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–70.
Zhang B, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204:49–55.
Chew V, et al. Immune activation underlies a sustained clinical response to yttrium-90 radioembolisation in hepatocellular carcinoma. Gut. 2019;68:335–46.
Giannini EG, et al. Overview of immune checkpoint inhibitors therapy for hepatocellular carcinoma, and the ITA.LI.CA cohort-derived estimate of amenability rate to immune checkpoint inhibitors in clinical practice. Cancers. 2019;11:1689.
Gomaa AI, et al. Diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2009;15:1301–14.
Grieb BC, et al. Evolving landscape of systemic therapy for hepatocellular carcinoma: breakthroughs, toxicities, and future frontiers. Am Soc Clin Oncol Educ Book. 2019;39:248–60.
Marrero JA, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–50.
Ang C, et al. Prevalence of established and emerging biomarkers of immune checkpoint inhibitor response in advanced hepatocellular carcinoma. Oncotarget. 2019;10:4018–25.
Harding JJ, et al. Prospective genotyping of hepatocellular carcinoma: clinical implications of next-generation sequencing for matching patients to targeted and immune therapies. Clin Cancer Res. 2019;25:2116.
Ruiz de Galarreta M, et al. β-Catenin activation promotes immune escape and resistance to anti–PD-1 therapy in hepatocellular carcinoma. Cancer Discov. 2019;9:1124.
Topalian SL, et al. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–87.
Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74.
Lawrence MS, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–8.
Bauch K, et al. Value of sonography in assessing nodular changes of the thyroid in an endemic goiter area. Z Gesamte Inn Med. 1986;41:542–7.
Connelly TG, Goldstein AM, Yamada T. Influence of hypophysectomy and starvation on blood glucose levels in the newt, Notophthalmus viridescens. J Exp Zool. 1974;188:367–73.
Rizvi NA, et al. Cancer immunology: mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–8.
Riedl W, et al. Zika virus NS3 mimics a cellular 14-3-3-binding motif to antagonize RIG-I- and MDA5-mediated innate immunity. Cell Host Microbe. 2019;26:493–503.e6.
Zhang K, et al. The comparison of tumor mutational burden (TMB) in patients of early- and late-stage lung adenocarcinoma in China. J Clin Oncol. 2018;36:8520.
Neureiter D, et al. Hepatocellular carcinoma: therapeutic advances in signaling, epigenetic, and immune targets. World J Gastroenterol. 2019;25:3136–50.
Meyer MA, et al. Breast and pancreatic cancer interrupt IRF8-dependent dendritic cell development to overcome immune surveillance. Nat Commun. 2018;9:1250.
Ng CKY, et al. Circulating cell-free DNA in hepatocellular carcinoma: current insights and outlook. Front Med. 2018;5:78.
Jiang P, et al. Lengthening and shortening of plasma DNA in hepatocellular carcinoma patients. Proc Natl Acad Sci U S A. 2015; 112:E1317–25.
Chan KC, et al. Cancer genome-scanning in plasma: detection of tumor-associated copy number aberrations, single-nucleotide variants, and tumoral heterogeneity by massively parallel sequencing. Clin Chem. 2013;59:211–24.
Ng CKY, et al. Genetic profiling using plasma-derived cell-free DNA in therapy-naive hepatocellular carcinoma patients: a pilot study. Ann Oncol. 2018;29:1286–91.
Friemel J, et al. Intratumor heterogeneity in hepatocellular carcinoma. Clin Cancer Res. 2015;21:1951–61.
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60.
Miller A, et al. Reporting results of cancer treatment. Cancer. 1981;47:207–14.
Therasse P, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–16.
Xu W, et al. Immunotherapy for hepatocellular carcinoma: recent advances and future perspectives. Ther Adv Med Oncol. 2019;11:1758835919862692.
Suzuki C, et al. Radiologic measurements of tumor response to treatment: practical approaches and limitations. RadioGraphics. 2008;28:329–44.
Seymour L, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–52.
Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36.
Llovet JM, et al. Design and end points of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711.
Shim JH, et al. Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? A validation study of old and new models. Radiology. 2012;262:708–18.
Kim BK, et al. Prospective comparison of prognostic values of modified Response Evaluation Criteria in Solid Tumours with European Association for the Study of the Liver criteria in hepatocellular carcinoma following chemoembolisation. Eur J Cancer. 2013;49:826–34.
Gillmore R, et al. EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol. 2011;55:1309–16.
Hussein RS, Tantawy W, Abbas YA. MRI assessment of hepatocellular carcinoma after locoregional therapy: insights into. Imaging. 2019;10:8.
European Association for the Study of the Liver. Electronic address, e.e.e. and L. European Association for the Study of the, EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236.
Roberts LR, et al. Imaging for the diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis. Hepatology. 2018;67:401–21.
Kloeckner R, et al. MDCT versus MRI assessment of tumor response after transarterial chemoembolization for the treatment of hepatocellular carcinoma. Cardiovsc Intervent Radiol. 2010;33:532–40.
Yaghmai V, et al. Imaging assessment of hepatocellular carcinoma response to locoregional and systemic therapy. Am J Roentgenol. 2013;201:80–96.
Rimola J. Heterogeneity of hepatocellular carcinoma on imaging. Semin Liver Dis. 2020;40:61–9.
Iqbal SI, Stuart KE. Assessment of tumor response in patients receiving systemic and nonsurgical locoregional treatment of hepatocellular cancer. UpToDate. Waltham: UpToDate, 2018.
Hayano K, Lee SH, Sahani DV. Imaging for assessment of treatment response in hepatocellular carcinoma: current update. Indian J Radiol Imaging. 2015;25:121–8.
Morsbach F, et al. Computed tomographic perfusion imaging for the prediction of response and survival to transarterial radioembolization of liver metastases. Invest Radiol. 2013;48:787–94.
Chen G, et al. Computed tomography perfusion in evaluating the therapeutic effect of transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2008;14:5738–43.
Popovic P, et al. Computed tomographic perfusion imaging for the prediction of response and survival to transarterial chemoembolization of hepatocellular carcinoma. Radiol Oncol. 2017;52:14–22.
Su T-H, et al. Early response of hepatocellular carcinoma to chemoembolization: volume-computed tomography liver perfusion imaging as a short-term response predictor. J Comput Assist Tomogr. 2017;41:315–20.
Tamandl D, et al. Early response evaluation using CT perfusion one day after transarterial chemoembolization for HCC predicts treatment response and long-term disease control. Eur J Radiol. 2017;90:73–80.
Loffroy R, et al. Intraprocedural C-arm dual-phase cone-beam CT: can it be used to predict short-term response to TACE with drug-eluting beads in patients with hepatocellular carcinoma? Radiology. 2013;266: 636–48.
Altenbernd J, et al. Treatment response after radioembolisation in patients with hepatocellular carcinoma: an evaluation with dual-energy computed tomography. Eur J Radiol Open. 2016;3:230–5.
Coenegrachts K, et al. Improved focal liver lesion detection: comparison of single-shot diffusion-weighted echoplanar and single-shot T2-weighted turbo spin echo techniques. Br J Radiol. 2007;80:524–31.
Piana G, et al. New MR imaging criteria with a diffusion-weighted sequence for the diagnosis of hepatocellular carcinoma in chronic liver diseases. J Hepatol. 2011;55:126–32.
Kamel IR, et al. Unresectable hepatocellular carcinoma: serial early vascular and cellular changes after transarterial chemoembolization as detected with MR imaging. Radiology. 2009;250:466–73.
Yu JI, et al. The role of diffusion-weighted magnetic resonance imaging in the treatment response evaluation of hepatocellular carcinoma patients treated with radiation therapy. Int J Radiat Oncol Biol Phys. 2014;89:814–21.
Schraml C, et al. Navigator respiratory‐triggered diffusion‐weighted imaging in the follow-up after hepatic radiofrequency ablation: initial results. J Magnet Resonance Imaging. 2009;29:1308–16.
Taouli B, et al. Hepatocellular carcinoma: perfusion quantification with dynamic contrast-enhanced MRI. Am J Roentgenol. 2013;201:795–800.
Su Y, et al. The efficacy and safety of dendritic cells co-cultured with cytokine-induced killer cell therapy in combination with TACE-predominant minimally invasive treatment for hepatocellular carcinoma: a meta-analysis. Clin Lab. 2016;62:599–608.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Woeste, M.R., Geller, A.E., Martin, R.C.G. et al. Optimizing the Combination of Immunotherapy and Trans-Arterial Locoregional Therapy for Stages B and C Hepatocellular Cancer. Ann Surg Oncol 28, 1499–1510 (2021). https://doi.org/10.1245/s10434-020-09414-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-09414-5