Abstract
Background
Anticancer drugs generate excessive reactive oxygen species (ROS), which can cause cell death. Cancer cells can resist this oxidative stress, but the mechanism of resistance and associations with chemoresistance are unclear. Here, we focused on Sirtuin 3 (SIRT3), a deacetylating mitochondrial enzyme, in oxidative stress resistance in colorectal cancer (CRC).
Methods
To evaluate SIRT3-related changes in mitochondrial function, ROS (mtROS) induction, and apoptosis, we used the human CRC cell lines HT29 and HCT116 transfected with short-hairpin RNA targeting SIRT3 and small interfering RNAs targeting superoxide dismutase 2 mitochondrial (SOD2) and peroxisome proliferator–activated receptor γ coactivator-1 (PGC-1α). In 142 clinical specimens from patients with CRC, we also assessed the association of SIRT3 protein levels (high/low) and prognosis.
Results
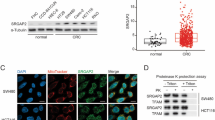
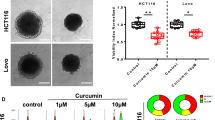
SIRT3 expression correlated with mtROS generation and apoptosis induction in cells treated with anticancer agents. Suppressing SIRT3 increased mtROS levels and cell sensitivity to anticancer agents. SIRT3 knockdown decreased SOD2 expression and activity, and suppressing SOD2 also improved sensitivity to anticancer drugs. In addition, SIRT3 was recruited with PGC-1α under oxidative stress, and suppressing SIRT3 decreased PGC-1α expression and mitochondrial function. PGC-1α knockdown decreased mitochondrial activity and increased apoptosis in cells treated with anticancer drugs. In resected CRC specimens, high vs low SIRT3 protein levels were associated with significantly reduced cancer-specific survival.
Conclusions
SIRT3 expression affected CRC cell chemoresistance through SOD2 and PGC-1α regulation and was an independent prognostic factor in CRC. SIRT3 may be a novel target for CRC therapies and a predictive marker of sensitivity to chemotherapy.





Similar content being viewed by others
References
Colvin H, Mizushima T, Eguchi H, Takiguchi S, Doki Y, Mori M. Gastroenterological surgery in Japan: the past, the present and the future. Ann Gastroenterol Surg. 2017;1(1):5–10.
Takeda M, Koseki J, Takahashi H, et al. Disruption of endolysosomal RAB5/7 efficiently eliminates colorectal cancer stem cells. Cancer Res. 2019;79(7):1426–37.
Hammond WA, Swaika A, Mody K. Pharmacologic resistance in colorectal cancer: a review. Ther Adv Med Oncol. 2016;8(1):57–84.
Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453–62.
Tada-Oikawa S, Oikawa S, Kawanishi M, Yamada M, Kawanishi S. Generation of hydrogen peroxide precedes loss of mitochondrial membrane potential during DNA alkylation-induced apoptosis. FEBS Lett. 1999;442(1):65–9.
Murata M, Suzuki T, Midorikawa K, Oikawa S, Kawanishi S. Oxidative DNA damage induced by a hydroperoxide derivative of cyclophosphamide. Free Radic Biol Med. 2004;37(6):793–802.
Kitahara T, Haraguchi N, Takahashi H, et al. Identification and characterization of CD107a as a marker of low reactive oxygen species in chemoresistant cells in colorectal cancer. Ann Surg Oncol. 2017;24(4):1110–9.
Liou G-Y, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44(5):479–96.
Someya S, Yu W, Hallows WC, et al. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143(5):802–12.
Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12(6):662–7.
Torrens-Mas M, Hernández-López R, Oliver J, Roca P, Sastre-Serra J. Sirtuin 3 silencing improves oxaliplatin efficacy through acetylation of MnSOD in colon cancer. J Cell Physiol. 2018;233(8):6067–76.
Shi T, Wang F, Stieren E, Tong Q. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem. 2005;280(14):13560–7.
Vellinga TT, Borovski T, de Boer VC, et al. SIRT1/PGC1α-dependent increase in oxidative phosphorylation supports chemotherapy resistance of colon cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2015;21(12):2870–9.
Kim B, Jung JW, Jung J, et al. PGC1α induced by reactive oxygen species contributes to chemoresistance of ovarian cancer cells. Oncotarget. 2017;8(36):60299–311.
Guerra F, Arbini AA, Moro L. Mitochondria and cancer chemoresistance. Biochim Biophys Acta Bioenerg. 2017;1858(8):686–99.
Brierley JG, Gospodarowicz MK, Wittekind, C, editors. The TNM classification of malignant tumours. 8. Oxford: Wiley; 2017.
Zhang W, Hu X, Shen Q, Xing D. Mitochondria-specific drug release and reactive oxygen species burst induced by polyprodrug nanoreactors can enhance chemotherapy. Nat Commun. 2019;10(1):1704.
Xiong Y, Wang M, Zhao J, et al. SIRT3 is correlated with the malignancy of non-small cell lung cancer. Int J Oncol. 2017;50(3):903–10.
Cui Y, Qin L, Wu J, et al. SIRT3 enhances glycolysis and proliferation in SIRT3-expressing gastric cancer cells. PLoS One. 2015;10(6):e0129834.
Huang S, Chen X, Zheng J, et al. Low SIRT3 expression contributes to tumor progression, development and poor prognosis in human pancreatic carcinoma. Pathol Res Pract. 2017;213(11):1419–23.
Dong XC, Jing LM, Wang WX, Gao YX. Down-regulation of SIRT3 promotes ovarian carcinoma metastasis. Biochem Biophys Res Commun. 2016;475(3):245–50.
Liu C, Huang Z, Jiang H, Shi F. The sirtuin 3 expression profile is associated with pathological and clinical outcomes in colon cancer patients. Biomed Res Int. 2014;2014:871263.
Rowe LA, Degtyareva N, Doetsch PW. DNA damage-induced reactive oxygen species (ROS) stress response in Saccharomyces cerevisiae. Free Radic Biol Med. 2008;45(8):1167–77.
Mattiazzi M, Vijayvergiya C, Gajewski CD, et al. The mtDNA T8993G (NARP) mutation results in an impairment of oxidative phosphorylation that can be improved by antioxidants. Hum Mol Genet. 2004;13(8):869–79.
Coppedè F, Migliore L. DNA damage in neurodegenerative diseases. Mutat Res. 2015;776:84–97.
Nomura A, Dauer P, Gupta V, et al. Microenvironment mediated alterations to metabolic pathways confer increased chemo-resistance in CD133+ tumor initiating cells. Oncotarget. 2016;7(35):56324–37.
Mizutani S, Miyato Y, Shidara Y, et al. Mutations in the mitochondrial genome confer resistance of cancer cells to anticancer drugs. Cancer Sci. 2009;100(9):1680–7.
Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23(1):27–47.
DeBerardinis RJ, Chandel NS. Fundamentals of cancer metabolism. Sci Adv. 2016;2(5):e1600200.
Cluntun AA, Lukey MJ, Cerione RA, Locasale JW. Glutamine metabolism in cancer: understanding the heterogeneity. Trends Cancer. 2017;3(3):169–80.
Lukey MJ, Katt WP, Cerione RA. Targeting amino acid metabolism for cancer therapy. Drug Discov Today. 2017;22(5):796–804.
Tseng AH, Shieh SS, Wang DL. SIRT3 deacetylates FOXO3 to protect mitochondria against oxidative damage. Free Radic Biol Med. 2013;63:222–34.
Tseng AH, Wu LH, Shieh SS, Wang DL. SIRT3 interactions with FOXO3 acetylation, phosphorylation and ubiquitinylation mediate endothelial cell responses to hypoxia. Biochem J. 2014;464(1):157–68.
Ferber EC, Peck B, Delpuech O, Bell GP, East P, Schulze A. FOXO3a regulates reactive oxygen metabolism by inhibiting mitochondrial gene expression. Cell Death Differ. 2012;19(6):968–79.
Marinkovic D, Zhang X, Yalcin S, et al. Foxo3 is required for the regulation of oxidative stress in erythropoiesis. J Clin Investig. 2007;117(8):2133–44.
Olmos Y, Valle I, Borniquel S, et al. Mutual dependence of Foxo3a and PGC-1alpha in the induction of oxidative stress genes. J Biol Chem. 2009;284(21):14476–84.
Borniquel S, García-Quintáns N, Valle I, et al. Inactivation of Foxo3a and subsequent downregulation of PGC-1 alpha mediate nitric oxide-induced endothelial cell migration. Mol Cell Biol. 2010;30(16):4035–44.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Masakatsu Paku, Naotsugu Haraguchi, Mitsunobu Takeda, Shiki Fujino, Takayuki Ogino, Norikatsu Myoshi, Hidekazu Takahashi, Mamoru Uemura, Tsunekazu Mizushima, Hirofumi Yamamoto, Yuichiro Doki, and Hidetoshi Eguchi declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig.
1. (a, b) The level of mtROS and apoptosis was evaluated in human CRC cells with the treatment of anticancer agents. The positive rates of (a) MitoSOX Red and (b) Annexin V were assessed in each cell line exposed to 5-FU (10 μM), OXA (10 μM), and SN-38 (0.2 μM) for 48 h. CRC: colorectal cancer; mtROS: mitochondrial reactive oxygen species; 5-FU: 5-fluorouracil; OXA: oxaliplatin; SN-38: 7-ethyl-10-hydroxycamptothecin (TIFF 2197 kb)
Supplemental Fig.
2. The sensitivity to 5-FU, OXA, and SN-38 in human CRC cells was assessed by CCK-8 assay. Relative absorbance at 450 nm for each anticancer drug tested at 10 dilutions. CRC: colorectal cancer; 5-FU: 5-fluorouracil; OXA: oxaliplatin; SN-38: 7-ethyl-10-hydroxycamptothecin; CCK-8: Cell Counting Kit-8 (TIFF 2197 kb)
Supplemental Fig.
3. (a, b) The value of mtROS and apoptosis was assessed in the control cells and the shSIRT3-#2 cells with the treatment of anticancer agents. (a) The fluorescence intensities of MitoSOX Red and (b) Annexin V were higher in the shSIRT3-#2 cells exposed to 5-FU (10 μM), OXA (10 μM), or SN-38 (0.2 μM) for 48 h. (c) The sensitivity to H2O2 in the control cells and the shSIRT3-#2 cells was measured by CCK-8 assay. Relative absorbance at 450 nm for each anticancer agent tested at 10 dilutions in the control cells and the shSIRT3 cells. The shSIRT3-#2 cells were more sensitive to each agent than the control cells. CRC: colorectal cancer; mtROS: mitochondrial reactive oxygen species; 5-FU: 5-fluorouracil; OXA: oxaliplatin; SN-38: 7-ethyl-10-hydroxycamptothecin; CCK-8: Cell Counting Kit-8; shSIRT3: shRNA targeting SIRT3 (TIFF 2197 kb)
Supplemental Fig.
4. (a) The fluorescence intensity of MitoTracker Green was lower in the shSIRT3-#2 cells exposed to 5-FU (10 μM), OXA (10 μM), or SN-38 (0.2 μM) for 48 h. (b, c) The values for mitochondrial activity and apoptosis were assessed in the control cells and the siPGC-1α cells with the treatment of anticancer agents. The fluorescence intensities of (a) MitoTracker Green and (b) Annexin V were lower in the siPGC-1α cells exposed to 5-FU (10 μM), OXA (10 μM), or SN-38 (0.2 μM) for 48 h. CRC: colorectal cancer; 5-FU: 5-fluorouracil; OXA: oxaliplatin; SN-38: 7-ethyl-10-hydroxycamptothecin; shSIRT3: shRNA targeting SIRT3; siPGC-1α: PGC-1α siRNA (TIFF 2197 kb)
Supplemental Fig.
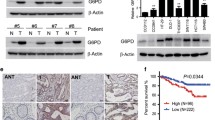
5. (a) The expression levels of SIRT3 were detected in the 13 human CRC frozen tissue samples from the 155 samples we collected and used for immunohistochemistry: weakly positive (n = 3), moderately positive (n = 4), strongly positive (n = 6). Weakly positive human CRC samples had low SIRT3 expressions, and SIRT3 expressions increased as the staining intensity increased. CRC: colorectal cancer (TIFF 2197 kb)
Rights and permissions
About this article
Cite this article
Paku, M., Haraguchi, N., Takeda, M. et al. SIRT3-Mediated SOD2 and PGC-1α Contribute to Chemoresistance in Colorectal Cancer Cells. Ann Surg Oncol 28, 4720–4732 (2021). https://doi.org/10.1245/s10434-020-09373-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-09373-x