Abstract
Background
Targeted axillary dissection (TAD) involves sentinel lymph node biopsy (SLNB) and excision of a biopsy-proven node marked by a clip. This study evaluates the feasibility of non-radioactive wireless localizers for targeted excision of clipped axillary lymph nodes.
Methods
We identified biopsy-proven, node-positive breast cancer patients treated with neoadjuvant therapy (NAT) and TAD from 2016 to 2020, and included those with a clipped node localized using SAVI SCOUT, Magseed, or RFID Tag. Primary outcome measures were (1) successful localization (ultrasound or mammographic-guided placement < 10 mm from target), and (2) retrieval of the clipped node during TAD, documented by specimen radiography or gross visualization. Secondary outcomes included rates of completion axillary lymph node dissection (cALND) and complications.
Results
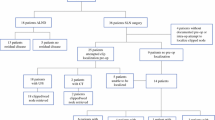
Overall, 57 patients were included; 1 (1.8%) patient had no clip visible at the time of localization, and no radiographic confirmation of clip placement at the time of biopsy, and was therefore excluded. In the remaining 56 patients, localization was successful in 53 (94.6%) patients and the clipped node was retrieved during TAD in 51 (91.1%) patients. Twenty-three of 27 (85.2%) ypN0 patients were spared cALND; 3 (11.1%) patients had cALND for failed clipped node retrieval during TAD, and 1 (3.7%) for false-positive frozen section. In patients with TAD alone, the rates of axillary seroma and infection were 20.0% and 8.6%, respectively.
Conclusions
Wireless non-radioactive localizers are feasible for axillary localization after NAT, with high success rates of retrieving clipped nodes. The lack of signal decay is an advantage of these devices, allowing flexibility in timing of placement.

Similar content being viewed by others
References
Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–72.
Alvarado R, Yi M, Le-Petross H, et al. The role for sentinel lymph node dissection after neoadjuvant chemotherapy in patients who present with node-positive breast cancer. Ann Surg Oncol. 2012;19(10):3177–84.
Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310(14):1455–61.
Mamtani A, Barrio AV, King TA, et al. How Often Does Neoadjuvant Chemotherapy Avoid Axillary Dissectino in Patients With Histologically Confirmed Nodal Metastases? Results of a Prospective Study. Ann Surg Oncol. 2016;23(11):3467–74.
Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015;33(3):258–64.
Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14(7):609–18.
Boughey JC, Ballman KV, Le-Petross HT, McCall LM, Mittendorf EA, Ahrendt GM, et al. identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0-T4, N1-N2) who receive neoadjuvant chemotherapy: results from ACOSOG Z1071 (Alliance). Ann Surg. 2016;263(4):802–7.
Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of a targeted axillary dissection. J Clin Oncol. 2016;34(10):1072–8.
Donker M, Straver ME, Wesseling J, et al: Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann Surg 2015;261:378–382.
Diego EJ, McAuliffe PF, Soran A, et al. Axillary staging after neoadjuvnt chemotherapy for breast cancer: a pilot study combining sentinel lymph node biopsy with radioactive seed localization of pre-treatment positive axillary lymph nodes. Ann Surg Oncol 2016;23(5):1549–53.
Nguyen TT, Hieken TJ, Glazebrook KN, et al. Localizing the clipped node in patients with node-positive breast cancer treated with neoadjuvant chemotherapy: early learning experience and challenges. Ann Surg Oncol 2017;24(10):3011–16.
Plecha D, Bai S, Patterson H, et al. Improving the accuracy of axillary lymph node surgery in breast cancer with ultrasound-guided wire localization of biopsy proven metastatic lymph nodes. Ann Surg Oncol 2015;22:4241–6.
Cianna Medical. “SCOUT Radar Localization for Breast Cancer Surgery”. https://www.ciannamedical.com/savi-scout/. Accessed 25 Apr 2020.
Devicor Medical Products, Inc. “Magseed”. https://www.mammotome.com/magseed/. Accessed 25 Apr 2020.
Faxitron Bioptics, LLC. “LOCalizer”. https://www.faxitron.com/product/localizer/. Accessed 25 Apr 2020.
Dauphine C, et al. A prospective clinical study to evaluate the safety and performance of wireless localization of nonpalpable breast lesions using radiofrequency identification technology. Am J Roentgenol. 2015;204(6):W720-3.
Reicher JJ, et al. Radiofrequency identification tags for preoperative tumor localization: proof of concept. Am J Roentgenol. 2008;191(5):1359–65.
DiNome ML, et al. Microchipping the breast: an effective new technology for localizing non-palpable breast lesions for surgery. Breast Cancer Res Treat. 2019;175:165–70.
McGugin C, Spivey T, Coopey S, Smith B, Kelly B, Gadd M, et al. Radiofrequency identification tag localization is comparable to wire localization for non-palpable breast lesions. Breast Cancer Res Treat. 2019;177(3):735–739. https://doi.org/10.1007/s10549-019-05355-0.
Caudle AS, Yang WT, Mittendorf EA, Black DM, Hwang R, Hobbs B, Hunt KK, Krishnamurthy S, Kuerer HM. Selective surgical localization of axillary lymph nodes containing metastases in patients with breast cancer: a prospective feasibility trial. JAMA Surg. 2015;150(2):137–43.
Price ER, Khoury AL, Esserman LJ et al. Initial clinical experience with an inducible magnetic seed system for preoperative breast lesion localization. AJR Am J Roentgenol 2018;210(4):913–7.
Harvey JR, Lim Y, Murphy J, et al. Safety and feasibility of breast lesion localization using magnetic seeds (Magseed): a multi-centre, open-label cohort study. Breast Cancer Res Treat 2018;169(3):531–6.
Hersi AF, Eriksson S, Ramos J, et al. A combined, totally magnetic technique with magnetic marker for non-palpable tumour localizatino and superparamagnetic iron oxide nanoparticles for sentinel lymph node detection in breast cancer surgery. Eur J Surg Oncol 2019;45(4):544–9.
Lamb LR, Bahl M, Specht MC, D’Alessandro HA, Lehman CD. Evaluation of a nonradioactive magnetic marker wireless localization program. AJR Am J Roentgenol 2018;211(4):940–5.
Cox CE, Russel S, Prowler V et al. A prospective, single arm, multi-site, clinical evaluation of nonradioactive surgical guidance technology for the location of nonpalpable breast lesions during excision. Ann Surg Oncol. 2016;23(10):3168–74.
Patel SN, Mango VL, Jadeja P et al. Reflector-guided breast tumor localization versus wire localization for lumpectomies: a comparison of surgical outcomes. Clin Imaging 2016;47:14–17.
Taback B, Jadeja P, Ha R. Enhanced axillary evaluation using reflector-guided sentinel lymph node biopsy: a prospective feasibility study and comparison with conventional lymphatic mapping techniques. Clin Breast Cancer. 2018;18(5):e869–74.
Greenwood HI, Wong JM, Mukhatar RA, Alvarado MD, Price ER. Feasibility of magnetic seeds for preoperative localization of axillary lymph nodes in breast cancer treatment. AJR Am J Roentgenol. 2019;213(4):953–957.
Devicor Medical Products, Inc. “Breast Biopsy Markers—HydroMARK”. https://www.mammotome.com/breast-biopsy-markers/hydromark/. Accessed 25 Apr 2020.
Siso C, de Torres J, Esgueva-Colmenarejo A, et al. Intraoperative ultrasound-guided excision of axillary clip in patients with node-positive breast cancer treated with Neaodjuvant therapy (ILINA Trial): a new tool to guide the excision of the clipped node after Neoadjuvant treatment. Ann Surg Oncol 2018;25(3):784–91.
Choudhery S, Simmons C, Harper L, Lee CU. Tomosynthesis-guided needle localization of breast and axillary lesions: our initial experience. AJR Am J Roentgenol 2019;212(4):943–6.
Wilke LG, McCall L, Posther KE et al. Surgical complications associated with sentinel lymph node biopsy: results from a prospective international cooperative group trial. Ann Surg Oncol 2006;13(4):491–500.
Lucci A, McCall LM, Beitsch et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the american college of surgeons oncology group Trial Z0011. J Clin Oncol 2007;25(24):3657–63.
Gunn J, Gibson T, Li Z et al. Symptomatic axillary seroma after sentinel lymph node biopsy: incidence and treatment. Ann Surg Oncol 2016;23(10):3347–53.
Moo TA, Edelweiss M, Hajiyeva S et al. Correction to: Is Low-Volume Disease in the Sentinel Node After Neoadjuvant Chemotherapy an Indication for Axillary Dissection? Ann Surg Oncol Epub 21 Feb 2020. https://doi.org/10.1245/s10434-020-08273-4.
Laws A, Hughes ME, Hu J et al. Impact of residual nodal disease burden on technical outcomes of sentinel lymph node biopsy for node-positive (cN1) breast cancer patients treated with Neoadjuvant chemotherapy. Ann Surg Oncol 2019;26(12):3846–55.
Komenka IK, Torabi R, Nair G et al. Intraoperative touch imprint and frozen section analysis of sentinel lymph nodes after neoadjuvant chemotherapy for breast cancer. Ann Surg 2010;251(2):319–22.
Alliance for Clinical Trials in Oncology. Comparison of axillary lymph node dissection with axillary radiation for patients with node-positive breast cancer treated with chemotherapy (ALLIANCE A011022). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01901094. Accessed 25 Apr 2020.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Alison Laws, Kayla Dillon, Bridget N. Kelly, Olga Kantor, Kevin S. Hughes, Michele A. Gadd, Barbara L. Smith, Leslie R. Lamb, and Michelle Specht have no disclosures to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Laws, A., Dillon, K., Kelly, B.N. et al. Node-Positive Patients Treated with Neoadjuvant Chemotherapy Can Be Spared Axillary Lymph Node Dissection with Wireless Non-Radioactive Localizers. Ann Surg Oncol 27, 4819–4827 (2020). https://doi.org/10.1245/s10434-020-08902-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-08902-y