Abstract
Purpose
To evaluate the oncologic efficacy and feasibility of nephron-sparing surgery (NSS) in adult Xp11.2 translocation renal cell carcinoma (RCC).
Patients and Methods
Seventy patients with Xp11.2 translocation RCC and 273 with conventional RCC from five institutions in Nanjing were retrospectively studied. All patients were older than 18 years and were categorized into clinical T1 (cT1) stage using preoperative imaging. Using the preoperative imaging and electronic medical records, anatomical and pathological features were collected and analyzed.
Results
Among patients with Xp11.2 translocation RCC, 18/36 (50.0%) with cT1a and 12/34 (35.3%) with cT1b tumors underwent NSS. The respective proportions in the conventional RCC group were 121/145 (83.4%) and 93/128 (72.7%). Among cT1a tumors, the Xp11.2 translocation RCCs tended to be adjacent to the collecting system, sinus, and axial renal midline compared with conventional RCCs. Patients with Xp11.2 translocation RCCs who underwent NSS had comparable progression-free survival (PFS) and overall survival to radical nephrectomy (RN) patients (P > 0.05). Among cT1b tumors, surgical margin positivity and pelvicalyceal, vascular, and region lymphatic involvement were more likely to occur in the Xp11.2 translocation RCCs (P < 0.05). Patients with Xp11.2 translocation RCC who underwent RN had a more favorable PFS than those who underwent NSS (P = 0.048). However, multivariate analysis of PFS did not identify surgical method as a risk factor (P = 0.089).
Conclusions
Among adults with Xp11.2 translocation RCC, NSS can be an alternative for patients with cT1a tumor but should be performed with more deliberation in patients with cT1b tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Xp11.2 translocation renal cell carcinoma (RCC) is a rare type of RCC associated with balanced translocation of transcription factor E3 (TFE3) and other fusion partners. The World Health Organization recognized Xp11.2 translocation RCC as a distinctive RCC entity in 20041 and reclassified it into microphthalmia transcription factor family translocation RCCs in 2016.2 Xp11.2 translocation RCC shows a more invasive course and more aggressive biological behavior than non-Xp11.2 translocation RCC,3,4 although Xp11 translocation RCC and clear cell RCC (ccRCC) have similar clinical courses.5 Up to half of patients with Xp11.2 translocation RCC present with regional progress or metastasis.6,7 Currently, complete excision is the most effective therapy for local Xp11.2 translocation RCC.
Radical nephrectomy (RN) remains the classical therapy with a reliable oncologic outcome for local RCC. By complete resection of the primary tumor and maximal preservation of the tumor-bearing kidney, nephron-sparing surgery (NSS) could realize oncologic efficacy as well as reduction in complications.8 Current guidelines recommend elective NSS as the standard surgical treatment for T1a renal tumors and favor NSS over RN for T1b tumors when technically feasible.9 However, studies have reported varied oncologic outcomes of NSS in diverse histological subtypes.10,11 Systematic reports on oncologic outcomes of Xp11.2 translocation RCC after NSS are still lacking. Due to the hysteresis of pathological diagnosis, an increasing number of Xp11.2 translocation RCCs at clinical T1 (cT1) stage underwent NSS, the same surgical strategy as that for conventional RCC. Considering the aggressive biological behavior of Xp11.2 translocation RCC, we designed a multicenter study to investigate the feasibility of NSS for Xp11.2 translocation RCC based on the most comprehensive clinical data.
Patients and Methods
Study Design and Patient Selection
The present retrospective study was approved by the institutional review board and performed in accordance with the ethical standards established by the five institutions. Altogether, 8598 cases of RCC with detailed clinicopathological data were reported between January 2007 and December 2019. Among these, 108 cases were eventually diagnosed as Xp11.2 translocation RCC by immunohistochemical staining12 and fluorescence in situ hybridization at five institutions.13,14 Clinicopathological data of patients with conventional RCC from these institutions during the same period were also randomly collected and used as controls (Fig. 1). Eligibility criteria for inclusion in the study were patients older than 18 years, with a solitary tumor at cT1 stage, a normal contralateral kidney, integrated imaging data, accurate pathological diagnosis, and a satisfactory follow-up. cT1 tumor was defined as a tumor ≤ 7 cm in the greatest dimension and limited to the kidney based on preoperative computed tomography (CT) imaging data. After verification of the eligibility criteria, 343 patients with cT1 tumors were centrally randomized and retrospectively studied. These included 70 patients with Xp11.2 translocation RCC and 273 with conventional RCC (Supplementary Material 1). The latter group comprised 167 patients with ccRCC, 63 with papillary RCC (pRCC), 30 with chromophobe RCC (chRCC), and 13 with clear cell papillary RCC.
Assessment of Variables
Clinical data including epidemiological features (sex and age), clinical manifestations, preoperative CT imaging (tumor size, laterality, and RENAL score), surgical methods (NSS or RN), clinical outcomes, and follow-up information were collected from the medical records. Detailed pathological features are an important reflection of tumor biological behavior. Information about pathological features including necrosis, sarcomatous, venous cancer thrombus, lymphatic metastasis, and other local invasion factors was also collected. Cases that underwent partial nephrectomy or tumor enucleation were included in the NSS group, while cases with complete removal of the affected kidney were included in the RN group.
According to the American Joint Committee on Cancer staging criteria (eighth edition, 2017), primary tumors were divided into the cT1a (≤ 4 cm in the greatest dimension, clinically limited to the kidney) group and the cT1b (> 4 cm and ≤ 7 cm in the greatest dimension, clinically limited to the kidney) group. Anatomical features are important factors that influence therapeutic strategies. The RENAL scores (Kutikov and Uzzo),15 which provide a standard way of describing anatomical features, were evaluated in consensus by a radiologist (Jian He) and a urologist (Feng Qu) based on patients’ preoperative CT imaging findings. The imaging data were reviewed on a picture archiving and communication system workstation (GE AW4.3; GE Healthcare, Chicago, IL). Survival data including progression-free survival (PFS) and overall survival (OS) were obtained from electronic medical records. PFS was defined as the time from the initiation of the surgery to the date of disease progression or censoring at the time of the last follow-up. OS was defined as the time interval between the date of the surgery and the date of death or the last follow-up.
Statistical Analysis
Patient baseline characteristics were analyzed using descriptive statistics. Continuous and categorical variables were analyzed using independent t test and Chi squared test, respectively. Univariate and multivariate Cox regression analyses were used to evaluate the predictive role of all factors for survival. PFS and OS curves were obtained by Kaplan–Meier analysis, and survival comparisons were performed using log-rank test. Statistical analyses were performed using IBM SPSS Statistics version 23.0 (IBM Corp., Armonk, NY), and the level of statistical significance was set at P < 0.05.
Results
Xp11.2 translocation RCCs accounted for 1.26% of all RCC cases. The mean age at onset of Xp11.2 translocation RCC was 32.2 ± 17.0 years, which was significantly lower than that of conventional RCC. There was a slight male predominance in the conventional RCC group, but this predominance was reversed in the Xp11.2 translocation RCC group (P < 0.001). Detailed clinicopathological characteristics of the 343 cases are summarized in Table 1. Among cT1a tumors, 18/36 (50.0%) cases of Xp11.2 translocation RCC and 121/145 (83.4%) cases of conventional RCC underwent NSS. Among cT1b tumors, NSS was performed in 12/34 (35.3%) cases of Xp11.2 translocation RCC and in 93/128 (72.7%) cases of conventional RCC. Thus, Xp11.2 translocation RCCs at cT1a stage were more likely to undergo RN than conventional RCCs (P < 0.05).
The RENAL scores of patients are presented in Table 2. Regardless of the stage (cT1a or cT1b), Xp11.2 translocation RCCs were located closer to the collecting system or the sinus than conventional RCCs. Among cT1a tumors, Xp11.2 translocation RCCs were more likely to cross the axial renal midline or to be located entirely between the polar lines. Among cT1b tumors, Xp11.2 translocation RCCs showed a higher rate of endophytic growth. These features collectively contributed to the higher total RENAL scores of Xp11.2 translocation RCC.
Xp11.2 translocation RCCs were more likely to show necrosis (Table 3). The incidence rate of undefined border and advanced Fuhrman grade was significantly higher in Xp11.2 translocation RCCs at cT1a stage than that in conventional RCCs. Moreover, surgical margin positivity, pelvicalyceal invasion, vascular invasion, and lymphatic metastasis were more common in the Xp11.2 translocation RCCs in the cT1b group.
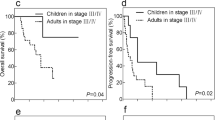
To explore the feasibility of NSS in Xp11.2 translocation RCC, patients were divided into two subgroups according to surgical method: in the RN subgroup, no significant differences were observed in PFS or OS between Xp11.2 translocation RCCs and conventional RCCs (Supplementary Material 2), whereas in the NSS subgroup, the PFS and OS of Xp11.2 translocation RCCs were significantly poorer than those of conventional RCCs (Supplementary Material 3). Among patients with Xp11.2 translocation RCC at cT1b stage, those who underwent RN had more favorable PFS than those who underwent NSS. However, there was no significant difference either in PFS or OS between surgical methods among patients with Xp11.2 translocation RCC at cT1a stage and all cT1 stage (Fig. 2). Cox proportional hazards analysis showed that surgical method failed to be accepted as a risk factor in the cT1a and the cT1b groups (Supplementary Material 4, 5).
Survival analysis of Xp11.2 translocation renal cell carcinoma with comparison between radical nephrectomy and nephron-sparing surgery subgroups: a overall and b progression-free survival of patients with cT1a tumor; c overall and d progression-free survival of patients with cT1b tumor; and e overall and f progression-free survival of patients both with cT1a and cT1b tumor
Discussion
The first case of Xp11.2 translocation RCC was reported in 1986 6,16 but the tumor was not described as a clinicopathological entity until 2001 and 2002.17,18 Recently, Xp11.2 translocation RCC has attracted increasing attention, and hundreds of cases have been reported. Among these, children and adults under 45 years of age were the most frequently affected. The incidence rate of Xp11.2 translocation RCC was one-third among pediatric patients with RCC and 15% among adult patients with RCC under 45 years of age.19,20 Due to the aggressive biological behavior and younger age at onset, many cases in early literature were found to be locally invasive at their primary diagnosis and thus underwent RN.6,7 With developments in the field of medicine, a growing number of small renal masses (including Xp11.2 translocation RCCs) were checked out, and a considerable number of these underwent NSS. However, literature regarding oncologic outcomes after NSS for Xp11.2 translocation RCCs is scarce, especially with respect to the adult population. To the best of our knowledge, the present study is the first to systematically compare the anatomic location, features of pathological invasion, and prognosis of Xp11.2 translocation RCCs and conventional RCCs at cT1a and cT1b stages.
With increasing size of tumor, the likelihood of local invasion, such as invasion into renal sinus, renal vein or its tributaries, capsule, and perinephric fat, increases sharply.21,22,–23 According to the study by Bonsib et al.,23 sinus invasion rate was 13% among ccRCCs with diameters smaller than 4 cm, 75% among those with diameters of 4.1–7 cm, and 97% among tumors larger than 7 cm. Besides, renal sinus involvement was associated with tumor histology, and its incidence was higher in ccRCC than in pRCC and chRCC.23 A considerable extent of local invasion was undetectable before the surgery, and patients subsequently underwent NSS.24,25 Our study revealed a higher local invasion rate in Xp11.2 translocation RCC, especially in patients with cT1b tumor. Altogether, 36.1% of the Xp11.2 translocation RCC at cT1a stage was undefined when compared with conventional RCC (12.4%). These results are consistent with the results reported by Cheng et al.,26 who reported that the incidence of pseudocapsule formation in Xp11.2 RCC was 63.6%.
Notably, lymphatic metastasis was common in Xp11.2 translocation RCC. This was observed in half of the patients with Xp11.2 translocation RCC at cT1b stage. A study by Ellis et al.,6 which is one of the biggest studies associated with regional lymph node involvement in Xp11.2 translocation RCC, reported that regional lymph node metastasis was observed in 24/32 (75%) of the ASPSCR1-TFE3 carcinomas and in 5/14 (35.7%) of the PRCC-TFE3 carcinomas. Taking all ASPSCR1-TFE3 and PRCC-TFE3 carcinomas into consideration, 7/16 (43.8%) T1a tumors and 6/12 (50.0%) T1b tumors showed regional lymph node involvement, which is consistent with our data. However, 11 out of 13 patients presenting with N1M0 disease remained disease-free in the short-term follow-up. Multivariate analysis showed that regional lymph node involvement did not portray a grim prognosis. Similar results were observed in the study by Galler et al.,27 wherein the majority of the patients with pediatric node-positive Xp11.2 translocation RCC survived without undergoing lymph node dissection. The authors proposed that “second look” lymph node dissections were not required for pediatric patients who did not undergo lymphadenectomy in the first procedure. However, it is unclear whether lymph node dissection was necessary for the treatment of Xp11.2 translocation RCC.
Pelvicalyceal invasion is another feature that needs attention. A high percentage of pelvicalyceal invasion in Xp11.2 translocation RCC is consistent with the common clinical presentation of gross hematuria.20,28 Due to the hollow structure of the renal pelvis, pelvicalyceal invasion is easier for localized RCC originating from the marginal parenchyma surrounding the renal pelvis.29,30 The anatomic features of endophytic growth, proximity to the collecting system, and central location relative to the polar lines observed in our study might explain the tendency of pelvicalyceal invasion in the Xp11.2 translocation RCC group. RCCs with pelvicalyceal invasion were possibly mistaken for transitional cell carcinoma, even after contrast-enhanced CT, CT urography, or magnetic resonance imaging (MRI).31,32 Four cases of Xp11.2 translocation RCC invading the renal pelvis in the present study initially mimicked transitional cell carcinoma and underwent ureteronephrectomy including cuff resection of the bladder wall. In the present study, none of the Xp11.2 translocation RCCs extended into the ureter or the bladder, which was different from the creeping feature of clear cell subtype observed in the dominant renal mass.30,33
A detailed understanding of the renal surgical anatomy is necessary while evaluating the feasibility of elective NSS. To date, the RENAL score designed by Kutikov et al. 15 is the most commonly used anatomical score system. The RENAL score has been revealed to be associated with histological features and aggressiveness.34,35 Renal lesions with low RENAL scores are usually associated with more indolent RCCs or benign histology.36,37 In addition, RENAL scores can predict postoperative recurrence and are negatively associated with OS.38,39 Our results showed that the “N” and the “L” categories of Xp11.2 translocation RCCs at cT1a stage tended to get a score of 3. However, the scores of conventional RCCs showed a relatively uniform distribution. Among patients with cT1b tumors, the “E” and the “N” scores of Xp11.2 translocation RCCs were higher than those of conventional RCCs. Thus, the anatomic location of Xp11.2 translocation RCC tended to be more central, which led to a higher total RENAL score. Therefore, the central location of Xp11.2 translocation RCCs made NSS difficult to perform, and RN was the surgical method of choice.
To date, only eight adult Xp11.2 translocation RCCs at pT1N0M0 stage (seven pT1a and one pT1b) have undergone NSS and maintained a stable course during a median follow-up of 13 and 37 months, respectively.40,41 In addition, 13 pediatric Xp11.2 translocation RCCs that underwent NSS have been reported. In the study by Ramphal,42 four pediatric Xp11.2 translocation RCCs underwent NSS and showed stable course with a median follow-up of 75 months. In the study by Liu et al.,43 nine children with tumor diameters less than 7 cm underwent NSS and achieved excellent outcomes, implying that NSS is an alternative treatment for pediatric Xp11.2 translocation RCCs measuring less than 7 cm. Unlike the indolent feature of pediatric cases, our results showed that, in adult Xp11.2 translocation RCCs, NSS achieved inferior oncologic outcomes than RN, even though the multivariate analysis of PFS did not identify surgical method as a risk factor. Nevertheless, NSS is an alternative for adult Xp11.2 translocation RCCs at cT1a stage.
As percutaneous puncture biopsy is not routinely performed for renal masses, Xp11.2 translocation RCCs are usually diagnosed postoperatively. In fact, Xp11.2 translocation RCC shares distinctive radiological features with conventional RCC, which could provide clues for the diagnosis of this rare tumor.44,45,46,–47 Typical dynamic CT features of Xp11.2 translocation RCC include cystic-solid renal masses with circular calcifications in the unenhanced phase and “slow-in and delay-out” pattern in the contrast-enhanced phases.44,45 MRI findings have revealed that Xp11.2 translocation RCCs are isointense on T1-weighted imaging, heterogeneously hypointense on T2-weighted imaging, and slightly hyperintense on diffusion-weighted imaging.46 On contrast-enhanced ultrasound, Xp11.2 translocation RCCs showed obvious hypoenhancement with irregular nonenhanced regions in the early phase and the delayed phases.47 Thus, radiologic imaging could be applied to differentiate Xp11.2 translocation RCC from conventional RCC. A risk-scoring system for preoperative diagnosis of adult Xp11.2 translocation RCC combining imaging with epidemiological characteristics is in progress. Reasonable preoperative planning could help optimize the operative techniques and improve the outcomes of Xp11.2 translocation RCC.
The present study has some limitations. Even though our study had the largest number of patients in the NSS group compared with previous studies, the number was still insufficient. The retrospective and multicenter design of our research led to many inherent biases, especially the selection bias. The low overall incidence of this type of RCC was the primary reason for these limitations. Further prospective studies with a greater number of NSS patients are required to confirm our findings.
In conclusion, our initial experience of a multicenter study in the Chinese population identified the central location of Xp11.2 translocation RCC. For cT1a tumors, the oncologic outcomes of NSS were comparable to those of RN. However, NSS is not recommended for cT1b tumors due to the possibility of increased risk of postoperative recurrence and metastasis. The efficacy and the feasibility of NSS in Xp11.2 translocation RCC warrant large-scale studies and long-term follow-up.
References
Lopez-Beltran A, Scarpelli M, Montironi R, Kirkali Z. 2004 WHO classification of the renal tumors of the adults. Eur Urol. 2006;49(5):798–805.
Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO classification of tumours of the urinary system and male genital organs-part A: renal, penile, and testicular tumours. Eur Urol. 2016;70(1):93–105.
Qiu R, Bing G, Zhou XJ. Xp11.2 Translocation renal cell carcinomas have a poorer prognosis than non-Xp11.2 translocation carcinomas in children and young adults: a meta-analysis. Int J Surg Pathol. 2010;18(6):458–64.
Xu L, Yang R, Gan W, et al. Xp11.2 translocation renal cell carcinomas in young adults. BMC Urol. 2015;15:57.
Sukov WR, Hodge JC, Lohse CM, et al. TFE3 rearrangements in adult renal cell carcinoma: clinical and pathologic features with outcome in a large series of consecutively treated patients. Am J Surg Pathol. 2012;36(5):663–70.
Ellis CL, Eble JN, Subhawong AP, et al. Clinical heterogeneity of Xp11 translocation renal cell carcinoma: impact of fusion subtype, age and stage. Mod Pathol. 2014;27(6):875–86.
7. Argani P, Olgac S, Tickoo SK, et al. Xp11 translocation renal cell carcinoma in adults: expanded clinical, pathologic, and genetic spectrum. Am J Surg Pathol. 2007;31(8):1149–60.
Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. 2011;59(4):543–52.
Ljungberg B, Albiges L, Abu-Ghanem Y, et al. European Association of Urology guidelines on renal cell carcinoma: The 2019 update. Eur Urol. 2019;75(5):799–810.
Yoo S, You D, Jeong IG, et al. Histologic subtype needs to be considered after partial nephrectomy in patients with pathologic T1a renal cell carcinoma: papillary vs. clear cell renal cell carcinoma. J Cancer Res Clin Oncol. 2017;143(9):1845–51.
Uzzo RG, Novick AC. Nephron sparing surgery for renal tumors: indications, techniques and outcomes. J Urol. 2001;166(1):6–18.
Argani P, Lal P, Hutchinson B, et al. Aberrant nuclear immunoreactivity for TFE3 in neoplasms with TFE3 gene fusions: a sensitive and specific immunohistochemical assay. Am J Surg Pathol. 2003;27(6):750–61.
Chen X, Yang Y, Gan W, et al. Newly designed break-apart and ASPL-TFE3 dual-fusion FISH assay are useful in diagnosing Xp11.2 translocation renal cell carcinoma and ASPL-TFE3 renal cell carcinoma: a STARD-compliant article. Medicine (Baltimore). 2015;94(19):e873.
Green WM, Yonescu R, Morsberger L, et al. Utilization of a TFE3 break-apart FISH assay in a renal tumor consultation service. Am J Surg Pathol. 2013;37(8):1150–63.
Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182(3):844–53.
de Jong B, Molenaar IM, Leeuw JA, Idenberg VJ, Oosterhuis JW. Cytogenetics of a renal adenocarcinoma in a 2-year-old child. Cancer Genet Cytogenet. 1986;21(2):165–9.
Argani P, Antonescu CR, Illei PB, et al. Primary renal neoplasms with the ASPL-TFE3 gene fusion of alveolar soft part sarcoma: a distinctive tumor entity previously included among renal cell carcinomas of children and adolescents. Am J Pathol. 2001;159(1):179–92.
Argani P, Antonescu CR, Couturier J, et al. PRCC-TFE3 renal carcinomas: morphologic, immunohistochemical, ultrastructural, and molecular analysis of an entity associated with the t(X;1)(p11.2;q21). Am J Surg Pathol. 2002;26(12):1553–66.
Cheng X, Gan W, Zhang G, Li X, Guo H. Clinical characteristics of XP11.2 translocation/TFE3 gene fusion renal cell carcinoma: a systematic review and meta-analysis of observational studies. BMC Urol. 2016;16(1):40.
Liu N, Wang Z, Gan W, et al. Renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusions: clinical features, treatments and prognosis. Plos One. 2016;11(11).
Taneja K, Arora S, Rogers CG, Gupta NS, Williamson SR. Pathological staging of renal cell carcinoma: a review of 300 consecutive cases with emphasis on retrograde venous invasion. Histopathology. 2018;73(4):681–91.
Williamson SR, Taneja K, Cheng L. Renal cell carcinoma staging: pitfalls, challenges, and updates. Histopathology. 2019;74(1):18–30.
Bonsib SM. T2 clear cell renal cell carcinoma is a rare entity: a study of 120 clear cell renal cell carcinomas. J Urol. 2005;174(4 Pt 1):1199–202; discussion 202.
Sokhi HK, Mok WY, Patel U. Stage T3a renal cell carcinoma: staging accuracy of CT for sinus fat, perinephric fat or renal vein invasion. Br J Radiol. 2015;88(1045):20140504.
Tsili AC, Argyropoulou MI. Advances of multidetector computed tomography in the characterization and staging of renal cell carcinoma. World J Radiol. 2015;7(6):110–27.
Cheng X, He J, Gan W, et al. Pseudocapsule of renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusion: a clue for tumor enucleation? Int J Clin Exp Pathol. 2015;8(5):5403–10.
Geller JI, Dome JS. Local lymph node involvement does not predict poor outcome in pediatric renal cell carcinoma. Cancer. 2004;101(7):1575–83.
Ma W, Liu N, Zhuang W, et al. Comparative clinicopathologic characteristics and outcomes of paediatric and adult Xp11 translocation renal cell carcinomas: a retrospective multicentre study in China. Sci Rep. 2020;10(1):2249.
Li Y, Ding YU, Chen D, et al. Renal cell carcinoma growing into the renal pelvis and mimicking transitional cell carcinoma: A case report and literature review. Oncol Lett. 2015;9(4):1869–72.
Chauhan NS, Bharti R, Chander B, Kumar S. Pediatric clear cell renal cell carcinoma with pelvicalyceal system invasion and contiguous extension up to bladder: novel and bizarre tumor behaviour. Pol J Radiol. 2016;81:256–60.
Kitazono MT, Coakley FV, Naeger DM, et al. CT of unusual renal masses invading the pelvicaliceal system: potential mimics of upper tract transitional cell carcinoma. Clin Imaging. 2011;35(1):77–80.
Bata P, Tarnoki DL, Tarnoki AD, et al. Transitional cell and clear cell renal carcinoma: differentiation of distinct histological types with multiphase CT. Acta Radiol. 2014;55(9):1112–9.
Gulati M, Gore JL, Pantuck AJ, et al. Ureteral tumor thrombus from renal cell carcinoma extending into bladder. Urol Oncol. 2007;25(5):393–5.
Wang HK, Zhu Y, Yao XD, et al. External validation of a nomogram using RENAL nephrometry score to predict high grade renal cell carcinoma. J Urol. 2012;187(5):1555–60.
Satasivam P, Sengupta S, Rajarubendra N, et al. Renal lesions with low R.E.N.A.L nephrometry score are associated with more indolent renal cell carcinomas (RCCs) or benign histology: findings in an Australian cohort. BJU Int. 2012;109 Suppl 3:44–7.
Mullins JK, Kaouk JH, Bhayani S, et al. Tumor complexity predicts malignant disease for small renal masses. J Urol. 2012;188(6):2072–6.
Kutikov A, Smaldone MC, Egleston BL, et al. Anatomic features of enhancing renal masses predict malignant and high-grade pathology: a preoperative nomogram using the RENAL Nephrometry score. Eur Urol. 2011;60(2):241–8.
Kopp RP, Mehrazin R, Palazzi KL, et al. Survival outcomes after radical and partial nephrectomy for clinical T2 renal tumours categorised by R.E.N.A.L. nephrometry score. BJU Int. 2014;114(5):708–18.
Nagahara A, Uemura M, Kawashima A, et al. R.E.N.A.L. nephrometry score predicts postoperative recurrence of localized renal cell carcinoma treated by radical nephrectomy. Int J Clin Oncol. 2016;21(2):367–72.
Gorin MA, Ball MW, Pierorazio PM, Argani P, Allaf ME. Partial nephrectomy for the treatment of translocation renal cell carcinoma. Clin Genitourin Cancer. 2015;13(3):e199-201.
Camparo P, Vasiliu V, Molinie V, et al. Renal translocation carcinomas: clinicopathologic, immunohistochemical, and gene expression profiling analysis of 31 cases with a review of the literature. Am J Surg Pathol. 2008;32(5):656–70.
Ramphal R, Pappo A, Zielenska M, Grant R, Ngan BY. Pediatric renal cell carcinoma: clinical, pathologic, and molecular abnormalities associated with the members of the mit transcription factor family. Am J Clin Pathol. 2006;126(3):349–64.
Liu C, Zhang W, Song H. Nephron-sparing surgery in the treatment of pediatric renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusions. J Pediatr Surg. 2017;52(9):1492–5.
He J, Gan W, Liu S, et al. Dynamic computed tomographic features of adult renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusions: comparison with clear cell renal cell carcinoma. J Comput Assist Tomogr. 2015;39(5):730–6.
He J, Zhou K, Zhu B, et al. Dynamic contrast-enhanced CT characterization of Xp11.2 translocation/TFE3 gene fusions versus papillary renal cell carcinomas. Biomed Res Int. 2015;2015:1–8.
Wang W, Ding J, Li Y, et al. Magnetic resonance imaging and computed tomography characteristics of renal cell carcinoma associated with Xp11.2 translocation/TFE3 gene fusion. PLoS One. 2014;9(6):e99990.
Ling W, Ma X, Luo Y, et al. Ultrasonographic findings of renal cell carcinomas associated with Xp11.2 translocation/TFE3 gene fusion. Contrast Media Mol Imaging. 2017;2017:2958357.
Acknowledgment
This research was supported by the National Natural Science Foundation of China (81572512), the Nanjing SCI-TECH Development Project (201803025), and the Beijing Ronghe Medical Development Foundation. The funders had no role in the study design, data collection or analysis, decision to publish, or preparation of the manuscript. The authors thank Jian He (Department of Radiology, Nanjing Drum Tower Hospital) for evaluation of the anatomical score. We also thank Jun Yang and Xiaohong Pu (Department of Pathology, Nanjing Drum Tower Hospital) for providing technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, N., Qu, F., Shi, Q. et al. Nephron-Sparing Surgery for Adult Xp11.2 Translocation Renal Cell Carcinoma at Clinical T1 Stage: A Multicenter Study in China. Ann Surg Oncol 28, 1238–1246 (2021). https://doi.org/10.1245/s10434-020-08813-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-08813-y