Abstract
Background
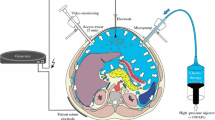
Pressurized intraperitoneal aerosol chemotherapy (PIPAC) is a new technology for delivering intraperitoneal chemotherapy. It is generally assumed that with PIPAC, the ratio of peritoneal to systemic drug concentration is superior to liquid hyperthermic intraperitoneal chemotherapy (HIPEC). To date, no direct comparative data are available supporting such an assumption.
Materials and Methods
Twelve 65-day-old pigs were randomly separated into three groups of four pigs each, all of which received intraperitoneal chemotherapy using the following administration methods: PIPAC with oxaliplatin 92 mg in 150 ml dextrose 5% (Group 1); PIPAC with electrostatic aerosol precipitation (ePIPAC; Group 2); or laparoscopic HIPEC (L-HIPEC) with oxaliplatin 400 mg in 4 L dextrose 5% at 42 °C (Group 3). Serial blood and peritoneal tissue concentrations of oxaliplatin were determined by spectrometry.
Results
In all three groups, the maximum concentration of oxaliplatin in blood was detected 50–60 min after onset of the chemotherapy experiments, with no significant differences among the three groups (p = 0.7994). Blood oxaliplatin concentrations (0–30 min) were significantly higher in the L-HIPEC group compared with the ePIPAC group (p < 0.05). No difference was found for the overall systemic oxaliplatin absorption (area under the curve). Overall concentrations in the peritoneum were not different among the three groups (p = 0.4725), but were significantly higher in the visceral peritoneum in the PIPAC group (p = 0.0242).
Conclusions
Blood and tissue concentrations were comparable between all groups; however, depending on the intraperitoneal area examined and the time points of drug delivery, the concentrations differed significantly between the three groups.





Similar content being viewed by others
References
Goodman MD, McPartland S, Detelich D, et al. Chemotherapy for intraperitoneal use: a review of hyperthermic intraperitoneal chemotherapy and early post-operative intraperitoneal chemotherapy. J Gastrointest Oncol. 2016;7:45–57.
Garofalo A, Valle M, Garcia J, et al. Laparoscopic intraperitoneal hyperthermic chemotherapy for palliation of debilitating malignant ascites. Eur J Surg Oncol. 2006;32:682–5.
Facchiano E, Scaringi S, Kianmanesh R, et al. Laparoscopic hyperthermic intraperitoneal chemotherapy (HIPEC) for the treatment of malignant ascites secondary to unresectable peritoneal carcinomatosis from advanced gastric cancer. Eur J Surg Oncol. 2008;34:154–8.
Valle M, Van der Speeten K, Garofalo A. Laparoscopic hyperthermic intraperitoneal peroperative chemotherapy (HIPEC) in the management of refractory malignant ascites: a multi-institutional retrospective analysis in 52 patients. J Surg Oncol. 2009;15:331–4.
Ba MC, Van der Speeten K, Garofalo A. Chemotherapy with laparoscope-assisted continuous circulatory hyperthermic intraperitoneal perfusion for malignant ascites. World J Gastroenterol. 2010;16:1901–7.
Ba MC, Long H, Zhang XL, et al. Laparoscopic hyperthermic intraperitoneal perfusion chemotherapy for patients with malignant ascites secondary to unresectable gastric cancer. J Laparoendosc Adv Surg Tech. 2016;26:32–9.
Solaß W, Hetzel A, Nadiradze G, et al. Description of a novel approach for intraperitoneal drug delivery and the related device. Surg Endosc. 2012;26:849–1855.
Solaß W, Kerb R, Mürdter T, et al. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol 2014;21:553–559.
Kakchekeeva T, Demtröder C, Herath NI, et al. In vivo feasibility of electrostatic precipitation as an adjunct to pressurized intraperitoneal aerosol chemotherapy (ePIPAC). Ann Surg Oncol. 2016;23:592–598.
Giger-Pabst U, Tempfer CB. How to perform safe and technically optimized pressurized intraperitoneal aerosol chemotherapy (PIPAC): experience after a consecutive series of 1200 procedures. J Gastrointest Surg. 2018;22:2187–2193.
Ferron G, Gesson-Paute A, Classe JM, et al. Feasibility of laparoscopic peritonectomy followed by intra-peritoneal chemohyperthermia: an experimental study. Gynecol Oncol. 2005;99:358–61.
Jacquet P, Sugarbaker PH. Current methodologies for clinical assessment of patients with peritoneal carcinomatosis. J Exp Clin Cancer Res. 1996;15:49–58.
Murono K, Kawai K, Hata K, et al. Regimens of intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal cancer. Anticancer Res. 2018;38:15–22.
Dedrick RL, Flessner MF. Pharmacokinetic problems in peritoneal drug administration: tissue penetration and surface exposure. J Natl Cancer Inst. 1997;89:480–487.
Solaß W, Hetzel A, Nadiradze G, et al. Intraoperative intraperitoneal drug delivery using a nebulizer: rationale and pharmacokinetic results. Surg Endosc. 2012;26:1849–1855.
Solass W, Herbette A, Schwarz T, et al. Therapeutic approach of human peritoneal carcinomatosis with Dbait in combination with capnoperitoneum: proof of concept. Surg Endosc. 2012;52:847–52.
Demtröder C, Solass W, Zieren J, Strumber D, Giger-Pabst U, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) with oxaliplatin in colorectal peritoneal metastasis. Colorectal Dis. 2016;18: 364–371.
Schilling MK, Redaelli C, Krahenbuhl L, et al. Splanchnic microcirculatory changes during CO2 laparoscopy. J Am Coll Surg. 1997;184:378–382.
Blanco A, Giger-Pabst U, Solass W, et al. Renal and hepatic toxicities after pressurized intraperitoneal aerosol chemotherapy (PIPAC). Ann Surg Oncol. 2013;20:2311–2316.
Göhler D, Khosrawipour V, Khosrawipour T, et al. Technical description of the microinjection pump (MIP®) and granulometric characterization of the aerosol applied for pressurized intraperitoneal aerosol chemotherapy (PIPAC). Surg Endosc. 2017;31:1778–1784.
Bellendorf A, Khosrawipour V, Khosrawipour T, et al. Scintigraphic peritoneography reveals a non-uniform 99mTc-Pertechnetat aerosol distribution pattern for pressurized intra-peritoneal aerosol chemotherapy (PIPAC) in a swine model. Surg Endosc. 2018;32:166–174.
Göhler D, Große S, Bellendorf A, et al. Hyperthermic intracavitary nanoaerosol therapy (HINAT) as an improved approach for pressurized intraperitoneal aerosol chemotherapy (PIPAC): Technical description, experimental validation and first proof of concept. Beilstein J Nanotechnol. 2017;18:2729–2740.
Acknowledgment
The authors are thankful to the staff of the CIRS Department at INRA Centre, Val de Loire, France, for their important support in performing the animal experiments. The authors are also deeply thankful to Julien Sobilo at CIPA-TAAM, CNRS Orleans, for data processing, and Katarzyna Gaj for her important secretarial assistance.
Funding
This study was funded by institutional funds.
Author information
Authors and Affiliations
Contributions
UG-P: Study design, animal experiments, data acquisition, data interpretation, manuscript drafting, and critical revision for important intellectual content of the manuscript. PB, ALP: Study design, study protocol, and critical revision for important intellectual content of the manuscript. SR, ALP, SL: Critical revision for important intellectual content of the manuscript. TAF: Animal experiments and data acquisition. NT: Data analysis and interpretation. CD: Critical revision for important intellectual content of the manuscript. ES: Critical revision for important intellectual content of the manuscript. MO: Study design, study protocol, animal experiments, data acquisition, data interpretation, manuscript drafting, and critical revision for important intellectual content of the manuscript.
Corresponding author
Ethics declarations
Disclosure
Urs Giger-Pabst, Petru Bucur, Sébastien Roger, Thomas Albert Falkenstein, Nicolas Tabchouri, Alain Le Pape, Stéphanie Lerondel, Cédric Demtröder, Ephrem Salamé, and Mehdi Ouaissi have no conflicts of interest to declare.
Availability of Data and Materials
The dataset of the current study is available from the corresponding author upon reasonable request.
Ethical Approval
The study protocol was approved by the local Animal Ethics Committee, Val de Loire, France.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Giger-Pabst, U., Bucur, P., Roger, S. et al. Comparison of Tissue and Blood Concentrations of Oxaliplatin Administrated by Different Modalities of Intraperitoneal Chemotherapy. Ann Surg Oncol 26, 4445–4451 (2019). https://doi.org/10.1245/s10434-019-07695-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07695-z