Abstract
Background
This study aimed to clarify differences in the prognostic impact of tumor spread through air spaces (STAS) in lobectomy versus sublobar resection (SR). The study also investigated the frequency and significance of STAS in residual lung segments.
Methods
This study identified 752 patients with p-stage 1A non-small cell lung cancer (NSCLC) from 2010 to 2012. Recurrence-free survival (RFS) and overall survival (OS) were compared. For proactive simulation of SR, 100 consecutive lobectomy specimens of p-stage 1A NSCLC were selected.
Results
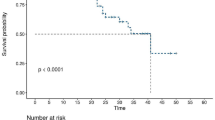
The study found STAS in 182 (28.7%) of 634 lobectomy cases and 43 (36.4%) of 118 SR cases. Multivariable analysis showed that STAS was not a prognostic factor in the lobectomy group, but showed a significantly worse prognostic effect for the SR group (RFS, P < 0.001; OS, P < 0.001). In 9 of 100 simulated cases, STAS occurred in residual lung segments. The patients with T1c category disease had a significantly increased risk for the development of STAS in residual lung segments (P = 0.033).
Conclusions
For patients with p-stage 1A lung cancer who have undergone SR, STAS is a prognostic indicator of poor outcomes. The presence of STAS does occasionally exist in the residual lung segments.


Similar content being viewed by others
References
Ramnath N, Dilling TJ, Harris LJ, et al. Treatment of stage III non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e314S–40S.
Ajmani GS, Wang CH, Kim KW, Howington JA, Krantz SB. Surgical quality of wedge resection affects overall survival in patients with early stage non-small cell lung cancer. J Thorac Cardiovasc Surg. 2018;156:380–391.e382.
Walter JE, Heuvelmans MA, de Jong PA, et al. Occurrence and lung cancer probability of new solid nodules at incidence screening with low-dose CT: analysis of data from the randomised, controlled NELSON trial. Lancet Oncol. 2016;17:907–16.
Smith CB, Kale M, Mhango G, et al. Comparative outcomes of elderly stage I lung cancer patients treated with segmentectomy via video-assisted thoracoscopic surgery versus open resection. J Thorac Oncol. 2014;9:383–9.
Fernando HC, Landreneau RJ, Mandrekar SJ, et al. Impact of brachytherapy on local recurrence rates after sublobar resection: results from ACOSOG Z4032 (Alliance), a phase III randomized trial for high-risk operable non-small cell lung cancer. J Clin Oncol. 2014;32:2456–62.
El-Sherif A, Fernando HC, Santos R, et al. Margin and local recurrence after sublobar resection of non-small cell lung cancer. Ann Surg Oncol. 2007;14:2400–5.
Sawabata N, Karube Y, Umezu H, et al. Cytologically malignant margin without continuous pulmonary tumor lesion: cases of wedge resection, segmentectomy, and lobectomy. Interactive Cardiovasc Thorac Surg. 2008;7:1044–8.
Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39–51.
Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol. 2011;24:653–64.
Hung JJ, Jeng WJ, Chou TY, et al. Prognostic value of the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification on death and recurrence in completely resected stage I lung adenocarcinoma. Ann Surg. 2013;258:1079–86.
Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10:1243–60.
WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. (4th ed.). http://apps.who.int/bookorders/anglais/detart1.jsp?codlan=1&codcol=70&codcch=4007.
Kadota K, Nitadori J-I, Sima CS, et al. Tumor spread through air spaces is an important pattern of invasion and impacts the frequency and location of recurrences after limited resection for small stage I lung adenocarcinomas. J Thorac Oncol. 2015;10:806–14.
Masai K, Sakurai H, Sukeda A, et al. Prognostic impact of margin distance and tumor spread through air spaces in limited resection for primary lung cancer. J Thorac Oncol. 2017;12:1788–97.
Shiono S, Endo M, Suzuki K, Yarimizu K, Hayasaka K, Yanagawa N. Spread through air spaces is a prognostic factor in sublobar resection of non-small cell lung cancer. Ann Thorac Surg. 2018;106:354–60.
Toyokawa G, Yamada Y, Tagawa T, et al. The significance of spread through air spaces in resected lung adenocarcinomas with lymph node metastasis. Clin Lung Cancer. 2018;19: 395–400.
Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition Lung Cancer Stage Classification. Chest. 2017;151:193–203.
Su H, Dai C, She Y, et al. Which T descriptor is more predictive of recurrence after sublobar resection: whole tumour size versus solid component size? Eur J Cardiothorac Surg. 2018;54:1028–36.
Landreneau RJ, Normolle DP, Christie NA, et al. Recurrence and survival outcomes after anatomic segmentectomy versus lobectomy for clinical stage I non-small-cell lung cancer: a propensity-matched analysis. J Clin Oncol. 2014;32:2449–55.
Kadota K, Kushida Y, Katsuki N, et al. Tumor spread through air spaces is an independent predictor of recurrence-free survival in patients with resected lung squamous cell carcinoma. Am J Surg Pathol. 2017;41:1077–86.
Dai C, Xie H, Su H, et al. Tumor spread through air spaces affects the recurrence and overall survival in patients with lung adenocarcinoma > 2 to 3 cm. J Thorac Oncol. 2017;12:1052–60.
Ettinger DS, Wood DE, Aisner DL, et al. Non-small cell lung cancer, version 5.2017, NCCN Clinical Practice Guidelines in Oncology. JNCCN J Natl Comprehensive Cancer Network. 2017;15:504–35.
Maurizi G, D’Andrilli A, Ciccone AM, et al. Margin distance does not influence recurrence and survival after wedge resection for lung cancer. Ann Thorac Surg. 2015;100:918–24; (discussion 924–5).
Gagné A, Racine É, Orain M, et al. Identification of grossing criteria for intraoperative evaluation by frozen section of lung cancer resection margins. Am J Surg Pathol. 2018;42:1495–502.
Morales-Oyarvide V, Mino-Kenudson M. Tumor islands and spread through air spaces: distinct patterns of invasion in lung adenocarcinoma. Pathol Int. 2016;66:1–7.
Blaauwgeers H, Flieder D, Warth A, et al. A prospective study of loose tissue fragments in non-small cell lung cancer resection specimens: an alternative view to “spread through air spaces.” Am J Surg Pathol. 2017;41:1226–30.
Higashiyama M, Kodama K, Takami K, Higaki N, Nakayama T, Yokouchi H. Intraoperative lavage cytologic analysis of surgical margins in patients undergoing limited surgery for lung cancer. J Thorac Cardiovasc Surg. 2003;125:101–7.
Acknowledgment
This study was supported by the projects from Shanghai Hospital Development Center (SHDC12015116), the Fundamental Research Funds for the Central Universities (22120180607), the National Natural Science Foundation of China (81802256), the Shanghai Lingjun Program (2015057), and the Shanghai Pujiang Program (15PJD034).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ren, Y., Xie, H., Dai, C. et al. Prognostic Impact of Tumor Spread Through Air Spaces in Sublobar Resection for 1A Lung Adenocarcinoma Patients. Ann Surg Oncol 26, 1901–1908 (2019). https://doi.org/10.1245/s10434-019-07296-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07296-w