Abstract
Purpose
The purpose of the study was to investigate the effect of spread through air spaces (STAS) on the postoperative prognosis of patients with multiple primary lung cancers staged from IA to IB based on tumor size.
Methods
Clinicopathological and follow-up data of 122 patients with multiple primary lung cancers diagnosed at stages IA-IB and surgically treated at the Department of Thoracic Surgery, Shenzhen people’s Hospital from January 2019 to December 2021 were retrospectively analyzed. The study involved 42 males and 80 females. STAS status was used to divide them into two groups (87 cases in STAS (-) and 35 cases in STAS (+)). A logistic regression analysis, univariate and multivariate Cox regression analysis, and Kaplan-Meier curves (K-M) were used to determine how STAS affected recurrence-free survival (RFS) in patients.
Results
STAS (+) had a significantly higher recurrence rate than STAS (-). STAS was predicted by smoking history (P = 0.044), main tumor diameter (P = 0.02), and solid nodules on chest CT (P = 0.02). STAS incidence was not significantly different between lobectomy and sublobar resection groups (P = 0.17). Solid nodules on CT, tumor diameter, vascular invasion, pleural invasion, and STAS were significant predictors of recurrence in the univariate Cox regression analysis. Tumor diameter, pleural invasion and STAS were significant prognostic factors for recurrence in the multivariate Cox regression analysis. Furthermore, STAS (+) group was at greater risk of recurrence than STAS (-) group (34% vs. 0%, P < 0.05)。.
Conclusion
Stage IA-IB multiple primary lung cancer patients with STAS (+) had a higher recurrence rate and a shorter overall survival rate.
Similar content being viewed by others
Introduction
As reported in the Global Cancer Report of 2022, the mortality rates for cancer have been consistently declining, with lung cancer showing the most significant decrease in mortality rates (In the past 30 years, lung cancer mortality rates have decreased by 56% in males and 32% in females). Even though lung cancer ranks among the most common cancer-related deaths globally, early detection and treatment are still vital [1]. More and more patients with lung nodules and even early-stage lung cancer are being detected by low-dose high-resolution computed tomography (CT) scans, increasing public awareness of multiple primary lung cancer (MPLC). The MPLC is the occurrence of two or more primary lung cancers in the same individual, either simultaneously or sequentially, located either in the same lung or in the contralateral lung, and they may have the same or different histological types [2]. MPLC is considered an early-stage lung cancer, and its prognosis is generally better, with surgical resection being the primary treatment modality [3].
The invasive behavior of lung cancer includes: (1) non-lepidic histological growth pattern; (2) fibroblastic proliferation with connective tissue growth; (3) invasion of blood vessels or the pleura. However, in 2015, the World Health Organization identified a fourth invasive behavior of lung cancer, spread through air space (STAS). The term is described as “A micropapillary cluster, compact group, or a single cancer cell spreads within air spaces beyond the initial tumor boundary into surrounding lung tissue.” [4]. The incidence of STAS in diagnosed lung cancer ranges from 15 to 73% based on relevant literature [5,6,7,8]. In meta-analyses, STAS has been demonstrated to be an important adverse prognostic factor for patients receiving surgical removal of lung cancer [9,10,11]. Furthermore, multiple studies have indicated a strong correlation between STAS and patient survival and prognosis, with STAS-positive patients exhibiting significantly shorter recurrence-free survival (RFS) compared to STAS-negative patients [12,13,14]. However, STAS is effective in the early days, whether the effect of RFS in lung adenocarcinoma is due to its concomitant adverse prognostic features and the different pathological diagnoses of tumor size for early lung adenocarcinoma has an effect.
To determine how STAS plays a role in MPLC prognosis and recurrence, our study examined the influence of STAS on the recurrence and metastasis of patients with multiple primary lung cancers based on a previously collected database.
Materials and methods
Database
It is a retrospective study based on the clinical medical database of Shenzhen People’s Hospital. Clinical and pathological TNM staging (8th edition of the International Union Against Cancer staging system) was used for staging. Data were collected on the following parameters: (1) patient characteristics like age, gender, smoking history, etc.; (2) diagnostic information, including diagnostic methods, thin-layer chest CT(The slice thickness of the CT was 1 mm), etc.; (3) surgical procedures, including lobectomy, segmentectomy, or wedge resection, etc.; (4) pathological findings, including size, grading, lymph node metastases, STAS, lymphatic vessel invasion, and pleural invasion, etc.; (5) results, including recurrence, mortality, and stability.
Data collection
General information: collected through the electronic medical record system, including surgical age, gender, smoking history, history of malignant tumors, and surgical approach.
Imaging information: collected from preoperative staging examinations, including chest CT, abdominal and urological ultrasound, positron emission tomography/computed tomography, bone scan.
Postoperative pathological information: Two pathologists with more than 3 years’ experience reviewed the slides. This includes tumor size, number of lesions, histological grade, adenocarcinoma subtypes, STAS (spread through air spaces), presence of mucinous adenocarcinoma, pleural invasion, vascular invasion, and neural invasion. In accordance with the 8th edition of the TNM staging system, we performed histological subtyping, grading, and pathological staging.
Patients
In the period January 2019 to December 2021, 122 patients diagnosed with lung cancer underwent lung cancer surgery .Inclusion criteria were as follows: (1) A single-center clinical and pathological study of patients who are eligible for surgery between 2019 and 2021 based on AJCC TNM staging (8th edition) for stage IA-IB non-small cell lung cancer (NSCLC ); (2) confirmation of NSCLC in at least two or more lesions by postoperative histology or cytology; (3) availability of complete preoperative imaging data and postoperative pathological reports; (4) availability of comprehensive follow-up information for the patients. The exclusion criteria were as follows: (1) evidence of intrapulmonary or distant lymph node metastasis based on postoperative pathology; (2) presence of concurrent malignancies in the patients; (3) coexistence of other serious diseases that may affect follow-up and significantly impact short-term survival; (4) receiving antitumor treatments such as chemotherapy, targeted therapy, or immunotherapy before surgery. MPLC was diagnosed based on the following criteria: (1) One or more lesions are adenocarcinomas in situ or minimally invasive adenocarcinomas; (2) presence of different histological types between the lesions; (3) presence of the same histological type with distinct cytological and stromal features among the lesions; (4) presence of the same histological type with consistent primary components (such as papillary or acinar) but differing in cytological and stromal features [15].
Statistical analysis
Various categorical and continuous variables were evaluated using Student’s t-tests. STAS was predicted by logistic regression analysis. All patients were followed up until September 2023. Time between surgery date and last appointment or recurrence was the primary endpoint. Recurrence-free survival was estimated using Kaplan-Meier methods. Prognostic factors were determined using a multivariate Cox proportional hazards regression analysis. Data analysis was performed using SPSS 26.0 software to assess survival differences. P-values less than 0.05 were considered statistically significant.
Results
A median age of 58 was observed at surgery (range 28 to 80). In total, 122 patients were enrolled, 42 of whom were males, and 80 of whom were females. There were 102 non-smokers (83.6%) and 20 smokers (16.4%). There were 113 cases in stage IA (92.7%) and 9 cases in stage IB (7.3%). There were 12 cases of vascular invasion (9.8%) and 13 cases of pleural invasion (10.6%). STAS was found in 35 cases (28.7%). Table 1 provides a summary of patients with STAS. In STAS patients, smokers, stage IB, solid nodules, vascular invasion, and pleural invasion was significantly higher. Multivariate analysis of factors influencing STAS before surgery (Table 2) showed that smoking history (P = 0.044), size of the main tumor diameter (P = 0.02), and solid nodules on chest CT (P = 0.02) were predictive factors for STAS.
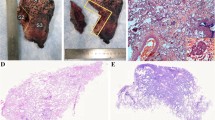
The 122 patients received 63 (51.6%) lobectomies and 59 (48.4%) sublobar resections (segmentectomy or wedge resection). Neither lobectomy nor sublobar resection showed a statistically significant difference in STAS incidence (P = 0.17). After lung cancer surgery, among the 122 patients, there were 12 cases of recurrence (10%) and 1 death (0.8%), all of which were in the STAS (+) group. The recurrence rate was 4.8% for lobectomy and 5.1% for sublobar resection, and there was no statistically significant difference between surgical procedures in terms of recurrence rates (P = 0.9). The sites of recurrence included 12 cases in the lungs, 2 cases in the bones, 3 cases in the lymph nodes, 1 case in the kidney, and 1 case in the pancreas. Based on Kaplan-Meier estimates, median follow-up was 33 months (95% confidence interval, 20–56 months). Overall recurrence-free survival was 65.7%. In univariate analysis, solid nodules on CT, tumor diameter, vascular invasion, pleural invasion, and STAS were significant predictors of recurrence (Table 3). Kaplan-Meier curve for recurrence-free survival is shown in Fig. 1; Recurrence-free survival was 65.7% for STAS positive patients and 100% for STAS negative patients (P<0 0.001). In the multivariate Cox regression analysis, tumor diameter, pleural invasion, and STAS played significant roles in predicting recurrence. (Table 3). Figure 2, 3 illustrates the CT images of three enrolled patients, and Figs. 4, 5 and 6 illustrates the pathological images of STAS of one enrolled patient.
Discussion
The study indicates that in MPLC patients, the STAS (+) group is more prone to recurrence compared to the STAS (-) group. Smoking history, size of the main tumor, and solid nodules on chest CT are predictive factors for STAS. Sublobar resection and lung lobectomy did not differ statistically in STAS incidence.
Multiple primary lung cancers are commonly adenocarcinomas, and distinguishing them from intrapulmonary metastases (IM) is often a concern for clinicians. In comparison to intrapulmonary metastases, multiple primary lung cancers generally have a better prognosis and a greater response to surgical resection, while intrapulmonary metastases are often advanced lesions with limited surgical options and are primarily treated with radiation, chemotherapy, and targeted therapy.
In terms of surgical approach, it is generally accepted that the physical squeezing pressure during surgery can affect the presence of STAS, especially in thoracoscopic surgery, when the specimen is removed through a tiny thoracoscopic hole. However, Blaauwgeers et al. were preceded by a prospective study [16]. It was shown that there was no difference in the incidence of loose fragments between different surgical groups (thoracotomy versus thoracoscopic surgery). Since STAS is associated with a higher ADC stage, the outcome may be due to the possibility that patients with a higher ADC stage may undergo radical surgery and thoracotomy. Whether STAS is a real in vivo phenomenon or an in vitro artifact is debatable. In thoracoscopic surgery, the excised lung specimen containing the tumor is squeezed through a tiny thoracoscopic foramen, which may cause tumor cells to detach around the tumor and move to adjacent air spaces [17]. In the current study, STAS is more common when thoracotomy is performed. In addition, numerous studies have shown that [18,19,20], STAS is independently associated with poor prognosis in patients with ADC. These arguments support that STAS is a real, important biological phenomenon and not a false artificial artifact.
Despite undergoing surgical treatment, 6.3-63.7% of MPLC patients still experience recurrence, suggesting the need to consider other risk factors for recurrence in addition to clinical staging [21]. Dai et al. suggested that STAS has a minimal impact on the prognosis of lung adenocarcinoma with a diameter < 2 cm, while STAS can significantly worsen the prognosis of patients with lung adenocarcinomas with diameters of 2–3 cm [22]. However, Kadota et al. found that STAS contributed independently to recurrence in lung adenocarcinoma with a 2 cm tumor diameter [23]. The studies primarily focused on early-stage lung adenocarcinoma, with limited experimental data regarding the relationship between STAS and MPLC.
Some studies propose that STAS is associated with epithelial-mesenchymal transition [24], which involves the loss of E-cadherin leading to decreased cell adhesion, promoting invasion and playing a role in tumor metastasis through lymphatic vessels, blood vessels, pleura, and STAS [25]. This mechanism may increase the likelihood of occult extrapulmonary metastasis during lung cancer surgery, increasing the risk of local and distant recurrence. STAS and its relationship with poor prognosis, particularly its association with IM, require further comprehensive research.
Moreover, considering that patients with multiple lung cancers require a greater extent of lung tissue resection, sublobar resection could be considered as the primary surgical approach to better preserve lung function. Suzuki et al. defined non-invasive lung cancer on imaging (tumor ≤ 2 cm, CTR ≤ 0.25) and found that sublobar resection with adequate margins (defined as at least 5 mm in the study) achieved close to 100% 5-year recurrence-free survival, with lower complication rates and less impact on lung function [26]. The studies primarily focused on patients with solitary lung cancer, and future research can focus on surgical approaches for multiple lung cancers.
Limitations
This study is based on comprehensive clinical follow-up data and provides a certain reference basis for clinical determination of diagnosis and treatment plans. However, it still has certain limitations. In this study, patients were followed up for only 3 years; Further surveillance of survival rates over five years and recurrence-free survival rates is needed. Moreover, this is a single-center retrospective study and the number of cases is little, which may have biases in patient selection. Further multicenter studies with larger sample sizes should be considered in the future.
Conclusions
In summary, in patients with MPLC, STAS is predominantly observed in invasive cases and is strongly associated with adverse prognosis and high rates of recurrence.
Data availability
No datasets were generated or analysed during the current study.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33.
Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg. 1975;70(4):606–12.
Stella F, Luciano G, Dell’ Amore A, Greco D, Ammari C, Giunta D, et al. Pulmonary metastases from NSCLC and MPLC (multiple primary lung cancers): management and outcome in a single centre experience. Heart Lung Circ. 2016;25(2):191–5.
Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to the 2015 World Health Organization Classification of Tumors of the lung, Pleura, Thymus, and heart. J Thorac Oncology: Official Publication Int Association Study Lung Cancer. 2015;10(9):1240–2.
Alvarez Moreno JC, Aljamal AA, Bahmad HF, Febres-Aldana CA, Rassaei N, Recine M, et al. Correlation between spread through air spaces (STAS) and other clinicopathological parameters in lung cancer. Pathol Res Pract. 2021;220:153376.
Xie H, Su H, Zhu E, Gu C, Zhao S, She Y, et al. Morphological subtypes of Tumor Spread through Air spaces in Non-small Cell Lung Cancer: prognostic heterogeneity and its underlying mechanism. Front Oncol. 2021;11:608353.
Tian Y, Feng J, Jiang L, Ning J, Gu Z, Huang J, et al. Integration of clinicopathological and mutational data offers insight into lung cancer with tumor spread through air spaces. Annals Translational Med. 2021;9(12):985.
Cao L, Jia M, Sun PL, Gao H. Histopathologic features from preoperative biopsies to predict spread through air spaces in early-stage lung adenocarcinoma: a retrospective study. BMC Cancer. 2021;21(1):913.
Chen D, Mao Y, Wen J, She Y, Zhu E, Zhu F, et al. Tumor Spread through Air spaces in Non-small Cell Lung Cancer: a systematic review and Meta-analysis. Ann Thorac Surg. 2019;108(3):945–54.
Wang S, Hao J, Qian C, Wang H. Tumor Spread through Air Spaces is a survival predictor in Non-small-cell Lung Cancer. Clin Lung Cancer. 2019;20(5):e584–e91.
Liu H, Yin Q, Yang G, Qie P. Prognostic impact of Tumor Spread through Air spaces in Non-small Cell Lung cancers: a Meta-Analysis including 3564 patients. Pathol Oncol Research: POR. 2019;25(4):1303–10.
Chen D, Wang X, Zhang F, Han R, Ding Q, Xu X, et al. Could tumor spread through air spaces benefit from adjuvant chemotherapy in stage I lung adenocarcinoma? A multi-institutional study. Therapeutic Adv Med Oncol. 2020;12:1758835920978147.
Mantovani S, Pernazza A, Bassi M, Amore D, Vannucci J, Poggi C, et al. Prognostic impact of spread through air spaces in lung adenocarcinoma. Interact Cardiovasc Thorac Surg. 2022;34(6):1011–5.
Zeng H, Tan FW, Yuan ZL, Ren JY, Xu JX, Xue Q. [Analysis of the effect of spread through air spaces on postoperative recurrence-free survival in patients with stage pT1N0M0 lung adenocarcinoma of different tumor size]. Zhonghua Yi Xue Za Zhi. 2022;102(19):1430–6.
Wang J, Zhang T, Ding H, Dong G, Xu L, Jiang F. [Research Progress in distinguishing methods of simultaneous multiple primary Lung Cancer and Intrapulmonary Metastasis]. Zhongguo Fei ai Za Zhi = Chinese. J lung cancer. 2021;24(5):365–71.
Blaauwgeers H, Flieder D, Warth A, et al. A prospective study of loose tissue fragments in non-small cell lung cancer resection specimens: an alternative view to spread through Air spaces. Am J Surg Pathol. 2017;41(9):1226–30.
Warth A. Spread through air spaces (STAS): a comprehensive update. Transl Lung Cancer Res. 2017;6(5):501–7.
Warth A, Muley T, Kossakowski CA, et al. Prognostic impact of intra alveolar tumor spread in pulmonary adenocarcinoma. Am J Surg Pathol. 2015;39(6):793–801.
Dai C, Xie H, Su H, et al. Tumor spread through air spaces affects therecurrence and overall survival in patients with lung adenocarcinoma > 2 to 3 cm. J Thorac Oncol. 2017;12(7):1052–60.
Han YB, Kim H, Mino-Kenudson M, et al. Tumor spread through air spaces (STAS): prognostic significance of grading in non-small cell lung cancer. Mod Pathol. 2021;34(3):549–61.
Hattori A, Takamochi K, Oh S, Suzuki K. Prognostic classification of multiple primary lung cancers based on a ground-glass opacity component. Ann Thorac Surg. 2020;109(2):420–7.
Dai C, Xie H, Su H, She Y, Zhu E, Fan Z, et al. Tumor Spread through Air Spaces affects the recurrence and overall survival in patients with lung adenocarcinoma > 2 to 3 cm. J Thorac Oncology: Official Publication Int Association Study Lung Cancer. 2017;12(7):1052–60.
Kadota K, Nitadori JI, Sima CS, Ujiie H, Rizk NP, Jones DR, et al. Tumor Spread through Air Spaces is an important pattern of Invasion and impacts the frequency and location of recurrences after Limited Resection for Small Stage I Lung Adenocarcinomas. J Thorac Oncology: Official Publication Int Association Study Lung Cancer. 2015;10(5):806–14.
Jung W, Chung JH, Yum S, Kim K, Lee CT, Jheon S, et al. The differential prognostic impact of spread through air spaces in early-stage lung adenocarcinoma after lobectomy according to the pT descriptor. J Thorac Cardiovasc Surg. 2022;163(1):277–84. e1.
Jin Y, Sun PL, Park SY, Kim H, Park E, Kim G, et al. Frequent aerogenous spread with decreased E-cadherin expression of ROS1-rearranged lung cancer predicts poor disease-free survival. Lung cancer (Amsterdam Netherlands). 2015;89(3):343–9.
Suzuki K, Watanabe SI, Wakabayashi M, Saji H, Aokage K, Moriya Y, et al. A single-arm study of sublobar resection for ground-glass opacity dominant peripheral lung cancer. J Thorac Cardiovasc Surg. 2022;163(1):289–301e2.
Acknowledgements
We sincerely thank the staff of Thoracic surgery in Shenzhen people’s Hospital for their support to this study.
Funding
This work was supported by Shenzhen Science and Technology R&D Fund (No. 20190801095354960).
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study. Materials were provided by Hong sheng Xie. Data collection was performed by Yuxin Wen, Shihua Dou, Xiaoxing Huang and Hong sheng Xie. Data analysis was conducted by Hong sheng Xie and reviewed by Hong sheng Xie and Lin Yang. The initial draft was written by Hong sheng Xie and reviewed by Hong sheng Xie and Lin Yang. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the (place name of institution and/or national research committee) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study has been approved by the Ethics Review Committee. The committee waived patient consent due to the nature of the study.
Consent for publication
We have obtained consent from all authors and participants, and they have agreed to publish the results of this study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xie, H., Dou, S., Huang, X. et al. The effect of spread through air spaces on postoperative recurrence-free survival in patients with multiple primary lung cancers. World J Surg Onc 22, 75 (2024). https://doi.org/10.1186/s12957-024-03351-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-024-03351-3