Abstract
Background
Immunotherapy targeting programmed cell death-1 (PD-1) and programmed death-ligand 1 (PD-L1) has shown dramatic therapeutic effects for lung squamous cell carcinoma (SCC), and PD-L1 expression has been shown not only to be a predictive biomarker for response to immunotherapy but also a prognostic factor for lung SCC. However, the clinical significance of programmed death-ligand 2 (PD-L2), another PD-1 ligand, remains unclear. Therefore, we analyzed PD-L2 expression by immunohistochemistry in surgically resected primary lung SCC.
Patients and Methods
PD-L1 and PD-L2 expression on tumor cells were analyzed in 211 primary lung SCC specimens by immunohistochemistry. Additionally, numbers of CD3+, CD4+, and CD8+ tumor-infiltrating lymphocytes were also examined.
Results
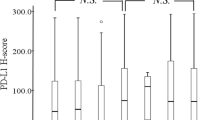
The rates of positive PD-L2 expression were 77.3% and 67.3% using 5% and 10% cut-off values, respectively. Low PD-L2 expression on tumor cells was statistically associated with histological type (non-keratinizing/keratinizing) and lymphatic invasion. PD-L2-positive patients had significantly longer postoperative survival time (log-rank test; p = 0.0170 at 5% cut-off and p = 0.0500 at 10% cut-off). Furthermore, survival analysis according to PD-L1 and PD-L2 expression revealed that PD-L1-positive and PD-L2-negative patients had the most unfavorable prognosis.
Conclusions
PD-L2 protein expression was associated with prognosis in primary lung SCC patients. PD-L2 expression might be a potential biomarker for response to PD-1/PD-L1-targeted immunotherapy, which should be investigated in future studies.


Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29.
Perez-Moreno P, Brambilla E, Thomas R, Soria JC. Squamous cell carcinoma of the lung: molecular subtypes and therapeutic opportunities. Clin Cancer Res. 2012;18(9):2443–51.
Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–39.
Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–35.
Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–33.
Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–65.
Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5(12):1365–9.
Ishida M, Iwai Y, Tanaka Y, et al. Differential expression of PD-L1 and PD-L2, ligands for an inhibitory receptor PD-1, in the cells of lymphohematopoietic tissues. Immunol Lett. 2002;84(1):57–62.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64.
Calles A, Liao X, Sholl LM, et al. Expression of PD-1 and its ligands, PD-L1 and PD-L2, in smokers and never smokers with KRAS-mutant lung cancer. J Thorac Oncol. 2015;10(12):1726–35.
Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2(3):261–8.
Yearley JH, Gibson C, Yu N, et al. PD-L2 Expression in human tumors: relevance to anti-PD-1 therapy in cancer. Clin Cancer Res. 2017;23(12):3158–67.
Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54.
Sacher AG, Gandhi L. Biomarkers for the Clinical Use of PD-1/PD-L1 inhibitors in non-small-cell lung cancer: a review. JAMA Oncol. 2016;2(9):1217–22.
Takada K, Okamoto T, Shoji F, et al. Clinical significance of PD-L1 protein expression in surgically resected primary lung adenocarcinoma. J Thorac Oncol. 2016;11(11):1879–90.
Takada K, Okamoto T, Toyokawa G, et al. The expression of PD-L1 protein as a prognostic factor in lung squamous cell carcinoma. Lung Cancer. 2017;104:7–15.
Kim MY, Koh J, Kim S, Go H, Jeon YK, Chung DH. Clinicopathological analysis of PD-L1 and PD-L2 expression in pulmonary squamous cell carcinoma: Comparison with tumor-infiltrating T cells and the status of oncogenic drivers. Lung Cancer. 2015;88(1):24–33.
Chen Z, Mei J, Liu L, et al. PD-L1 expression is associated with advanced non-small cell lung cancer. Oncol Lett. 2016;12(2):921–7.
Arrieta O, Montes-Servin E, Hernandez-Martinez JM, et al. Expression of PD-1/PD-L1 and PD-L2 in peripheral T-cells from non-small cell lung cancer patients. Oncotarget. 2017;8(60):101994–2005.
Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2(8):706–14.
Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26(2):259–71.
Mansfield AS, Aubry MC, Moser JC, et al. Temporal and spatial discordance of programmed cell death-ligand 1 expression and lymphocyte tumor infiltration between paired primary lesions and brain metastases in lung cancer. Ann Oncol. 2016;27(10):1953–8.
Tokito T, Azuma K, Kawahara A, et al. Predictive relevance of PD-L1 expression combined with CD8 + TIL density in stage III non-small cell lung cancer patients receiving concurrent chemoradiotherapy. Eur J Cancer. 2016;55:7–14.
Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800.
Azuma K, Ota K, Kawahara A, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol. 2014;25(10):1935–40.
Ota K, Azuma K, Kawahara A, et al. Induction of PD-L1 expression by the EML4-ALK oncoprotein and downstream signaling pathways in non-small cell lung Cancer. Clin Cancer Res. 2015;21(17):4014–21.
Shibahara D, Tanaka K, Iwama E, et al. Intrinsic and Extrinsic regulation of PD-L2 expression in oncogene-driven non-small cell lung cancer. J Thorac Oncol. 2018;13(7):926–37.
Youngnak P, Kozono Y, Kozono H, et al. Differential binding properties of B7-H1 and B7-DC to programmed death-1. Biochem Biophys Res Commun. 2003;307(3):672–7.
Cheng X, Veverka V, Radhakrishnan A, et al. Structure and interactions of the human programmed cell death 1 receptor. J Biol Chem. 2013;288(17):11771–85.
Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci USA. 2003;100(9):5336–41.
Hebenstreit D, Wirnsberger G, Horejs-Hoeck J, Duschl A. Signaling mechanisms, interaction partners, and target genes of STAT6. Cytokine Growth Factor Rev. 2006;17(3):173–88.
Zhang Y, Wang L, Li Y, et al. Protein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocarcinoma. OncoTargets Ther. 2014;7:567–73.
Hobo W, Maas F, Adisty N, et al. siRNA silencing of PD-L1 and PD-L2 on dendritic cells augments expansion and function of minor histocompatibility antigen-specific CD8 + T cells. Blood. 2010;116(22):4501–11.
Brown JA, Dorfman DM, Ma FR, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170(3):1257–66.
Akbari O, Stock P, Singh AK, et al. PD-L1 and PD-L2 modulate airway inflammation and iNKT-cell-dependent airway hyperreactivity in opposing directions. Mucosal Immunol. 2010;3(1):81–91.
Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–28.
Takamori S, Takada K, Toyokawa G, et al. PD-L2 expression as a potential predictive biomarker for the response to anti-PD-1 drugs in patients with non-small cell lung cancer. Anticancer Res. 2018;38(10):5897–901.
Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol. 2017;12(2):208–22.
Rimm DL, Han G, Taube JM, et al. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 Expression in non-small cell lung cancer. JAMA Oncol. 2017;3(8):1051–8.
Acknowledgement
The authors thank James P. Mahaffey, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10434_2019_7257_MOESM6_ESM.tif
Supplementary material 6 (TIFF 9525 kb). Supplementary Figure 1 A receiver operating characteristic curve in the analysis of enrolled patients. 5 year-survival was used as the state variable.
10434_2019_7257_MOESM7_ESM.tif
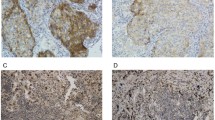
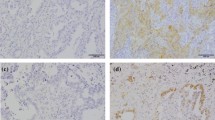
Supplementary material 7 (TIFF 18325 kb). Supplementary Figure 2. Representative images of PD-L1, PD-L2, CD3, CD4, and CD8 expression in lung squamous cell carcinoma specimens. Negative (A) and positive (B) PD-L1 staining, and negative (C) and positive (D) PD-L2 staining, which were detected on the membrane of tumor cells. Low (E, G, I) and high (F, H, J) numbers of CD3- (E, F), CD4- (G, H), and CD8-positive (I, J) tumor infiltrating lymphocytes. PD-L1, programmed death-ligand 1; PD-L2, programmed death-ligand 2; CD3, cluster of differentiation 3; CD4, cluster of differentiation 4; CD8, cluster of differentiation 8. Scale bar: 100 μm.
Rights and permissions
About this article
Cite this article
Matsubara, T., Takada, K., Azuma, K. et al. A Clinicopathological and Prognostic Analysis of PD-L2 Expression in Surgically Resected Primary Lung Squamous Cell Carcinoma. Ann Surg Oncol 26, 1925–1933 (2019). https://doi.org/10.1245/s10434-019-07257-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07257-3