Abstract
Background
c-Met relies on CD44v6 for its activation and signaling in several cancer cell lines. However, the correlation of c-Met and CD44v6 expression and its biological significance in esophageal squamous cell carcinoma (ESCC) remains unknown.
Methods
Expression of c-Met and CD44v6 was examined by immunohistochemistry (IHC) in 147 ESCC specimens. We analyzed the impact of c-Met and CD44v6 expression on clinicopathological parameters, including chemoresistance or prognosis in ESCC.
Results
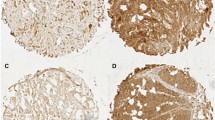
High expression of c-Met and CD44v6 in cancerous lesions was identified in 49.7% and 50.3% of all patients, respectively. The c-Met-high group comprised more advanced pT and pM stages than the c-Met-low group. In addition, more patients in the c-Met-high group received neoadjuvant chemotherapy (NACT) than the c-Met-low group (64.4% vs. 43.2%, P = 0.010). On the other hand, the CD44v6-high group was associated with more advanced pT/pN stages and a poorer clinical response to NACT (response rate 53.5% vs. 77.8%, P = 0.025) than the CD44v6-low group. Double-positive immunostaining of c-Met and CD44v6 was identified in 28.6% of all cases, and multivariate analysis of overall survival (OS) identified them (hazard ratio 1.79, 95% confidence interval 1.03–3.04, P = 0.038) as independent prognostic factors in addition to pN and pM stage.
Conclusions
c-Met/CD44v6 were associated with tumor progression or chemoresistance. Double-positive expression of c-Met and CD44v6 negatively impacted patient prognosis in ESCC, implying that c-Met and CD44v6 are candidates for targeted therapy in ESCC.



Similar content being viewed by others
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19(1):68–74.
Makino T, Doki Y, Miyata H, et al. Use of (18)F-fluorodeoxyglucose-positron emission tomography to evaluate responses to neo-adjuvant chemotherapy for primary tumor and lymph node metastasis in esophageal squamous cell carcinoma. Surgery. 2008;144(5):793–802.
Bahrami A, Shahidsales S, Khazaei M, et al. C-Met as a potential target for the treatment of gastrointestinal cancer: Current status and future perspectives. J Cell Physiol. 2017;232(10):2657–73.
Boccaccio C, Comoglio PM. Invasive growth: a MET-driven genetic programme for cancer and stem cells. Nat Rev Cancer. 2006;6(8):637–45.
Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11(12):834–48.
Tomihara H, Yamada D, Eguchi H, et al. MicroRNA-181b-5p, ETS1, and the c-Met pathway exacerbate the prognosis of pancreatic ductal adenocarcinoma after radiation therapy. Cancer Sci. 2017;108(3):398–407.
Kim JH, Kim BJ, Kim HS. Clinicopathological impacts of high c-Met expression in head and neck squamous cell carcinoma: a meta-analysis and review. Oncotarget. 2017;8(68):113120–8.
Wilson GD, Thibodeau BJ, Fortier LE, et al. Cancer stem cell signaling during repopulation in head and neck cancer. Stem Cells Int. 2016;2016:1894782.
Li C, Wu JJ, Hynes M, et al. c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology. 2011;141(6):2218–27 e2215.
Sun S, Wang Z. Head neck squamous cell carcinoma c-Met(+) cells display cancer stem cell properties and are responsible for cisplatin-resistance and metastasis. Int J Cancer. 2011;129(10):2337–48.
Bicaku E, Xiong Y, Marchion DC, et al. In vitro analysis of ovarian cancer response to cisplatin, carboplatin, and paclitaxel identifies common pathways that are also associated with overall patient survival. Br J Cancer. 2012;106(12):1967–75.
Aebersold DM, Kollar A, Beer KT, Laissue J, Greiner RH, Djonov V. Involvement of the hepatocyte growth factor/scatter factor receptor c-met and of Bcl-xL in the resistance of oropharyngeal cancer to ionizing radiation. Int J Cancer. 2001;96(1):41–54.
De Bacco F, Luraghi P, Medico E, et al. Induction of MET by ionizing radiation and its role in radioresistance and invasive growth of cancer. J Natl Cancer Inst. 2011;103(8):645–61.
Chen GZ, Dai WS, Zhu HC, et al. Foretinib enhances the radiosensitivity in esophageal squamous cell carcinoma by inhibiting phosphorylation of c-Met. J Cancer. 2017;8(6):983–92.
Scagliotti GV, Shuster D, Orlov S, et al. Tivantinib in combination with erlotinib versus erlotinib alone for EGFR-Mutant NSCLC: an exploratory analysis of the Phase 3 MARQUEE Study. J Thorac Oncol. 2018;13(6):849–54.
Pant S, Patel M, Kurkjian C, et al. A Phase II Study of the c-Met inhibitor tivantinib in combination with FOLFOX for the treatment of patients with previously untreated metastatic adenocarcinoma of the distal esophagus, gastroesophageal junction, or stomach. Cancer Invest. 2017;35(7):463–72.
Rimassa L, Abbadessa G, Personeni N, et al. Tumor and circulating biomarkers in patients with second-line hepatocellular carcinoma from the randomized phase II study with tivantinib. Oncotarget. 2016;7(45):72622–33.
Lai AZ, Abella JV, Park M. Crosstalk in Met receptor oncogenesis. Trends Cell Biol. 2009;19(10):542–51.
Tremmel M, Matzke A, Albrecht I, et al. A CD44v6 peptide reveals a role of CD44 in VEGFR-2 signaling and angiogenesis. Blood. 2009;114(25):5236–44.
Orian-Rousseau V, Chen L, Sleeman JP, Herrlich P, Ponta H. CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Dev. 2002;16(23):3074–86.
Matzke-Ogi A, Jannasch K, Shatirishvili M, et al. Inhibition of tumor growth and metastasis in pancreatic cancer models by interference with CD44v6 signaling. Gastroenterology. 2016;150(2):513–25 e510.
Snyder EL, Bailey D, Shipitsin M, Polyak K, Loda M. Identification of CD44v6(+)/CD24- breast carcinoma cells in primary human tumors by quantum dot-conjugated antibodies. Lab Invest. 2009;89(8):857–66.
Todaro M, Gaggianesi M, Catalano V, et al. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell. 2014;14(3):342–56.
Ozawa Y, Nakamura Y, Fujishima F, et al. c-Met in esophageal squamous cell carcinoma: an independent prognostic factor and potential therapeutic target. BMC Cancer. 2015;15:451.
Zhang T, Li H, Zhang Y, Wang P, Bian H, Chen ZN. Expression of proteins associated with epithelial-mesenchymal transition in esophageal squamous cell carcinoma. Oncol Lett. 2018;15(3):3042–8.
Yang H, Liu J, Yu H, et al. Expression and association of CD44v6 with prognosis in T2-3N0M0 esophageal squamous cell carcinoma. J Thorac Dis. 2014;6(2):91–8.
Ren JL, Wu HF, Wang WJ, et al. C-Met as a potential novel prognostic marker in squamous cell carcinoma and adenocarcinoma of esophagus: evidence from a meta-analysis. Panminerva Med. 2017;59(1):97–106.
Xu Y, Peng Z, Li Z, et al. Expression and clinical significance of c-Met in advanced esophageal squamous cell carcinoma. BMC Cancer. 2015;15:6.
Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumors. 7th edn. 2010, Oxford.
Yamashita K, Makino T, Yamasaki M, et al. Comparison of short-term outcomes between 2- and 3-field lymph node dissection for esophageal cancer. Dis Esophagus. 2017;30(11):1–8.
Miyata H, Yamasaki M, Miyazaki Y, et al. Clinical importance of supraclavicular lymph node metastasis after neoadjuvant chemotherapy for esophageal squamous cell carcinoma. Ann Surg. 2015;262(2):280–5.
Makino T, Yamasaki M, Miyata H, et al. Solitary Lymph node recurrence of esophageal squamous cell carcinoma: surgical failure or systemic disease? Ann Surg Oncol. 2016;23(6):2087–93.
Colvin H, Mizushima T, Eguchi H, Takiguchi S, Doki Y, Mori M. Gastroenterological surgery in Japan: the past, the present and the future. Ann Gastroenterol Surg. 2017;1(1):5–10.
Miyata H, Yamasaki M, Takahashi T, et al. Relevance of [18F]fluorodeoxyglucose positron emission tomography-positive lymph nodes after neoadjuvant chemotherapy for squamous cell oesophageal cancer. Br J Surg. 2013;100(11):1490–7.
Makino T, Yamasaki M, Tanaka K, et al. Metabolic tumor volume change predicts long-term survival and histological response to preoperative chemotherapy in locally advanced esophageal cancer. Ann Surg. 2018. https://doi.org/10.1097/SLA.0000000000002808
Diseases JSfE. Guidelines for the clinical and pathologic studies on carcinoma of the esophagus. 11th edn. 2015, Tokyo, Japan.
Xu YP, Lin G, Sun XJ, et al. C-Met as a molecular marker for esophageal squamous cell carcinoma and its association with clinical outcome. J Cancer. 2016;7(5):587–94.
Sierra JR, Tsao MS. c-MET as a potential therapeutic target and biomarker in cancer. Ther Adv Med Oncol. 2011;3(1 Suppl):S21–35.
Dulak AM, Stojanov P, Peng S, et al. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat Genet. 2013;45(5):478–86.
Bandla S, Pennathur A, Luketich JD, et al. Comparative genomics of esophageal adenocarcinoma and squamous cell carcinoma. Ann Thorac Surg. 2012;93(4):1101–6.
Chen C, Zhao S, Karnad A, Freeman JW. The biology and role of CD44 in cancer progression: therapeutic implications. J Hematol Oncol. 2018;11(1):64.
Hu B, Luo W, Hu RT, Zhou Y, Qin SY, Jiang HX. Meta-analysis of prognostic and clinical significance of CD44v6 in esophageal cancer. Medicine (Baltimore). 2015;94(31):e1238.
Rimassa L, Assenat E, Peck-Radosavljevic M, et al. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): a final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol. 2018;19(5):682–93.
Wang H, Jiang D, Song Q, et al. Prognostic impact and potential interaction of EGFR and c-Met in the progression of esophageal squamous cell carcinoma. Tumour Biol. 2016;37(7):9771–9.
Zheng B, Ni CH, Chen H, et al. New evidence guiding extent of lymphadenectomy for esophagogastric junction tumor: Application of Ber-Ep4 Joint with CD44v6 staining on the detection of lower mediastinal lymph node micrometastasis and survival analysis. Medicine (Baltimore). 2017;96(14):e6533.
Author information
Authors and Affiliations
Contributions
TH, TM, MY, MM, and YD: conception, design of the study; TH, TM, MY, KT, and YD: surgery and acquisition of clinical data; TH: immunohistochemical staining; TH, TM, and NM: evaluation of immunohistochemical staining; TH, TM, YM, TT, YK, and KN: analysis of data; TH: drafting the article; TM: critical revision of the article; MM and YD: final approval of the article.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. S1
Kaplan-Meier survival analysis of overall survival among patients who (a) did not receive preoperative therapy classified by c-Met expression, (b) did not receive preoperative therapy classified by CD44v6 expression, (c) did not receive preoperative therapy classified by c-Met and CD44v6 expression, (d) received preoperative chemotherapy classified by c-Met expression, (e) received preoperative chemotherapy classified by CD44v6 expression, and (f) received preoperative chemotherapy classified by c-Met and CD44v6 expression (TIFF 193 kb)
Supplemental Fig. S2
Kaplan-Meier survival analysis of overall survival according to c-Met/CD44v6 expression in (a) pT1-2 group, (b) pN0 group, (c) pT3-4 group, (d) pN1-3 group (TIFF 154 kb)
Rights and permissions
About this article
Cite this article
Hara, T., Makino, T., Yamasaki, M. et al. Effect of c-Met and CD44v6 Expression in Resistance to Chemotherapy in Esophageal Squamous Cell Carcinoma. Ann Surg Oncol 26, 899–906 (2019). https://doi.org/10.1245/s10434-018-07126-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-018-07126-5