Abstract
Introduction
The role of CD147 as an important indicator of tumor prognosis remains controversially discussed in literature. We focused on the prognostic significance of CD147 expression in esophageal cancer patients. While some studies report that CD147 is an unfavorable prognostic factor in esophageal squamous cell carcinoma, others showed no significant correlation. However, only one study draws attention to the significance of CD147 in esophageal adenocarcinoma, which is one of the most rapidly increasing neoplasms in the western world.
Methods
To finally clarify the impact of CD147 as a prognostic factor, especially for esophageal adenocarcinomas, we analyzed CD147 expression in a tissue microarray of 359 esophageal adenocarcinomas and 254 esophageal squamous cell cancer specimens. For the immuno-histochemical analysis, we used a primary antibody specific for CD147. Staining intensity and proportion of positive tumor cells were scored (negative, weak, moderate, strong staining). These findings were compared to normal esophageal tissue and correlated to the histopathological tumor phenotype and survival data.
Results
CD147 expression was detectable in weak intensities in benign esophageal tissue (85.78%) and expressed in predominately moderate to strong intensities in esophageal cancer (88.34%). Strong CD147 immunostaining was linked to increased infiltration depth (p = 0.015) and differentiation (p = 0.016) in esophageal squamous cell cancer but revealed no significant correlation with histopathology of adenocarcinoma. Moreover, CD147 intensity was unrelated to overall survival in this collective for both subtypes of esophageal cancer.
Conclusion
Thus, our data show that CD147 has no prognostic value, neither in esophageal adenocarcinoma nor squamous cell carcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of esophageal cancers has been increasing in recent years. One of the main causes of deaths is the fact, that clinical symptoms often only become apparent in late stages. There are marked geographical differences in the general incidence of esophageal carcinoma. In addition, esophageal cancer is one of the most aggressive types of carcinoma and it is the sixth leading cause of cancer death worldwide (Sung et al. 2021). A distinction is made between the two subtypes, esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC). In Western industrialized countries, the incidence of adenocarcinoma shows a significant and sustained rise over the last four decades, which makes esophageal cancer a major global health challenge (Smyth et al. 2017). Due to lack of early symptoms, patients are usually diagnosed in a locally advanced or metastasized stage, contributing to the fact that only about 40% of patients are suitable for surgery at the time of diagnosis. Thus, the 5-year overall survival rate is still poor (Sung et al. 2021). Identifying novel biological markers and tumorigenic pathways is vital for prediction of tumor behavior and may allow for personalized therapy.
The complex pathological process of tumor growth and spread includes several different pathways. Especially the interaction between tumor cells and host stromal cells is of particular interest. CD147, also known as extracellular matrix metalloproteinase inducer (EMMPRIN), is a widely expressed multifunctional glycoprotein (Biswas et al. 1995) and exerts essential roles in various cellular processes in several tissue types, such as the lung, thymus, retina, skin, cornea and nervous system (Iacono et al. 2007; Nabeshima et al. 2006). In various cancers, CD147 is highly expressed on the cell surface (Muramatsu and Miyauchi 2003; Yan et al. 2005; Yurchenko et al. 2006) and promotes the synthesis and secretion of matrix metalloproteinases in fibroblasts. This leads to degradation of the cancer cell matrix facilitating invasion and metastatic dissemination (Guo et al. 1997; Huang et al. 2015; Kanekura et al. 2002; Peng et al. 2016; Sameshima et al. 2000) on the one hand, and activation of tumor angiogenesis (Tang et al. 2004, 2005), and enhancement of cell survival signaling (Marieb et al. 2004) on the other. Several studies have described a correlation between increased CD147 expression and disease progression. Due to its membranous localization, CD147 has been suggested as a potential therapeutic target in several cancer types (Dean et al. 2009; Han et al. 2010; Hu et al. 2017; Zhang et al. 2018).
However, the prognostic role of CD147 in esophageal cancer remains controversial (Feng et al. 2013; Ishibashi et al. 2004; Wan and Wu 2012; Zhang et al. 2018; Zhu et al. 2011) with inconsistent results so far and nearly all available results reflecting patients with ESCC. The majority of studies suggest poor outcome for elevated CD147 expression (Huang et al. 2015; Wan and Wu 2012; Zhang et al. 2018; Zhu et al. 2011), whereas the largest cohort so far was not able to reveal any impact on recurrence-free survival (Ishibashi et al. 2004). Some studies linked high CD147 expression to advanced clinical stages (Huang et al. 2015; Wan and Wu 2012; Zhang et al. 2018) and lymph node metastasis (Feng et al. 2013; Huang et al. 2015; Wan and Wu 2012; Zhang et al. 2018; Zhu et al. 2011), while others reported no association with blood or lymphatic vessel invasion, tumor stage or distant metastasis (Cheng et al. 2006; Huang et al. 2015; Ishibashi et al. 2004). Some studies revealed an association between CD147 and invasion depth (Wan and Wu 2012; Zhu et al. 2011) and poor differentiation (Cheng et al. 2006; Feng et al. 2013; Huang et al. 2015; Wan and Wu 2012; Zhu et al. 2011), while others presented contradictory findings (Cheng et al. 2006; Ishibashi et al. 2004).
These controversies might be attributed to small and varying sample sizes and a limited level of evidence. To get more insights into the prognostic role of CD147 expression in esophageal cancer, especially in adenocarcinomas, we analyzed CD147 expression in a cohort of 359 adenocarcinomas and 254 squamous cell cancer specimens using a tissue microarray (TMA) with corresponding clinico-pathological data.
Materials and methods
Patients
Consecutive patients, which underwent radical esophagectomy at the Department of General, Visceral and Thoracic Surgery at the University Medical Center Hamburg-Eppendorf, were eligible for inclusion. Histopathological and clinical outcome data were collected and analyzed.
The tissue microarray technique was used to enable efficient analysis at the protein level. All specimens were analyzed according to a standard procedure, including complete embedding of the entire esophagus for histological analysis.
The study was approved by the Ethics Committee Hamburg and conducted in accordance with the Declaration of Helsinki. Usage of routinely archived formalin-fixed leftover patient tissue samples for research is approved by local laws and does not require written consent (HmbKHG, §12,1).
Tissue microarray manufacturing
The TMA manufacturing process, as described by Mirlacher et al., was used for our study (Mirlacher 2010; Simon et al. 2004). We removed a cylinder measuring 0.6 mm from each patient’s primary, representative, formalin-fixed, and paraffin-embedded tumor block. The cylinders were then assembled in an empty array receiver block (Simon et al. 2004). The tissues were distributed among two TMA blocks. For internal controls, both TMA blocks also contained various control tissues, including normal esophageal tissue.
Immunohistochemistry
For the immuno-histochemical analysis, all slides were de-paraffinized and exposed to heat-induced antigen retrieval for 5 min in an autoclave at 121 °C in pH 9 Tris–EDTA buffer. Next, a primary antibody specific for CD147 (mouse, monoclonal, Abcam #ab78106; at 1/150 dilution) was applied to the samples. All methods were carried out in accordance with the respective protocols. Visualization of the bound antibody was achieved with the EnVision Kit (Dako, Glostrup, Denmark). CD147 staining was found to be homogeneous in the analyzed tumor samples. Therefore, the tissue specimens were initially categorized semi-quantitatively into groups depending on CD147 expression.
The staining intensity (0, 1 + , 2 + , 3 +) and the proportion of positive tumor cells were analyzed separately for each tissue sample. These two parameters then served for final grading in negative, weak, moderate, and strong staining, as previously described by Juhnke et al. (2017). Negative scores indicated a complete absence of staining in the sample. A weak score was attributed when staining intensity was 1 + in up to 70% or 2 + in up to 30% of the tumor cells. Samples were classified as moderate with a staining intensity of 1 + in > 70%, 2 + in > 30% but in ≤ 70% or 3 + in ≤ 30% of the tumor cells. Strong scores showed a staining intensity of 2 + in > 70% or 3 + in > 30% of the tumor cells.
Statistical analysis
Statistical analyses were performed using JMP 9 (SAS Institute Inc., NC, USA). To recognize associations between molecular parameters and tumor phenotype, contingency tables, and the x2-test were used. Kaplan–Meier curves were generated for survival analysis. Testing for survival differences between groups was performed with the Log-Rank test. A two-sided p value < 0.05 was considered statistically significant.
Results
To elucidate the role of CD147, we analyzed cancer tissue from 359 esophageal adenocarcinoma patients and 254 esophageal squamous cell carcinoma patients.
Technical implications
A total of 300 of 359 (83.6%) adenocarcinomas and 217 of 254 (85.8%) squamous cell carcinomas were interpretable for our TMA analysis. Reasons for non-informative cases included a complete lack of tissue or absence of unequivocal cancer tissue in the TMA section.
Demographic and histopathological parameters
In the EAC group, 44 (14.7%) patients were female and 256 (85.3%) male. 67.0% were aged above 65 years. The majority of patients were detected in tumor stage pT3 (n = 180, 60.0%) and showed moderate (n = 114, 38.0%) to poor differentiation (n = 161, 53.7%).
In the ESCC group, tissue from 57 (26.3%) female and 160 (73.7%) male patients was available for analysis. 138 patients (63.5%) were older than 65 years and 123 (56.6%) were detected in tumor stage pT3. The largest share was moderately differentiated (n = 139, 64.0%). Median follow-up data for both groups were 17.3 (0–208; EAC) months and 12.2 (0–191; ESCC) months.
CD147 immunostaining in esophageal cancers
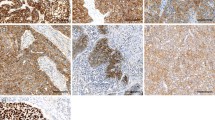
CD147 immunostaining was typically absent or detectable in weak intensities in benign esophageal tissue and was predominantly localized in the cell membrane. In esophageal cancer, CD147 expression was higher than in benign cells. CD147 immunostaining was detectable in weak intensities in 11.7%, moderate intensities in 41.7%, and strong intensities in 46.7% of EACs, while CD147 immunostaining was weak in 14.2%, moderate in 50.5%, and strong in 35.3% of ESCC specimens. Figure 1 shows representative images of weak and strong CD147 immunostaining in EAC and ESCC.
CD147 and histopathological tumor phenotype
For the subgroup of EAC, no significant correlation to infiltration depth (p = 0.513), lymphatic (p = 0.984) or distant dissemination (p = 0.643), tumor stage (p = 0.773) or differentiation (p = 0.135) was found (Table 1). Moreover, neither age nor gender had any impact on CD147 expression in these patients.
In the ESCC cohort however, infiltration depth (p = 0.015) and differentiation (p = 0.016) were significantly associated with CD147 expression. In contrast, lymphatic (p = 0.889) and distant metastasis (p = 0.918), tumor stage (p = 0.275), gender and age showed no significant correlation with CD147 expression (Table 2). For both subgroups, no association between CD147 and resection margins was apparent.
CD147 and overall survival
Follow-up data were available for 196 EAC and 167 ESCC patients with informative CD147 data. CD147 immunostaining was not linked to overall survival of EAC and ESCC patients (p = 0.9372 and p = 0.6919). The relationship between CD147 immunostaining and clinical outcome of the patients is demonstrated in Fig. 2.
Discussion
With these findings, we are able to clarify that CD147 has no prognostic relevance in esophageal adenocarcinoma or squamous cell cancer.
However, the comparative analysis of normal and neoplastic epithelium revealed an enhanced CD147 expression in most cancer specimens. While membranous CD147 was absent or weakly detectable in normal esophageal epithelium, strong staining was found in 46.7% EAC and 35.3% of ESCC patients. These results align with previous studies on CD147 expression in esophageal cancer, describing CD147 overexpression in malignant compared to non-malignant esophageal tissue (Cheng et al. 2006; Feng et al. 2013; Ishibashi et al. 2004; Wan and Wu 2012; Zhang et al. 2018; Zhu et al. 2011).
The upregulation of CD147 expression is supposedly associated with tumor progression in esophageal cancer (Landras et al. 2019). Numerous previous oncological studies dealt with CD147 due to its consistently high expression levels on the surface of various tumor types, such as malignant melanoma (Hu et al. 2017), colorectal (van der Jagt et al. 2006), breast (Marieb et al. 2004) and esophageal cancer (Cheng et al. 2006; Feng et al. 2013; Ishibashi et al. 2004; Wan and Wu 2012; Zhang et al. 2018; Zhu et al. 2011). CD147 has been shown to facilitate the secretion of extracellular matrix-degrading metalloproteases from fibroblasts, endometrial cells and cancer cells, such as MMP-1, MMP-3, MMP-9 and membrane type 1-MMP. In this way, carcinomatous cells can gain the ability to proliferate and migrate, promoting tumor growth and metastasis (Basset et al. 1990; Ellis et al. 1989; Xin et al. 2016).
Previously, the prognostic role of CD147 in esophageal cancer was controversially discussed in various studies (Feng et al. 2013; Ishibashi et al. 2004; Wan and Wu 2012; Zhang et al. 2018; Zhu et al. 2011). Almost all these studies reflected data from small numbers of esophageal squamous cell carcinoma patients. Since sample size is a significant factor in epidemiological studies, the limited cohort size in the above-mentioned studies might contribute to these controversies. In contrast, our cohort depicts the largest sample size so far, and our findings are in line with the largest currently published collective (Ishibashi et al. 2004), which did not find consideration in the meta-analysis.
In ESCC patients, we were able to find a significant correlation between CD147 expression, invasion depth and tumor differentiation, which is corroborated by previous studies (Cheng et al. 2006; Wan and Wu 2012; Zhu et al. 2011).
The only study dealing with esophageal adenocarcinoma, including 74 patients with type II/III adenocarcinoma of the esophagogastric junction (AEG) (Huang et al. 2015), also found a significantly higher rate of CD147 expression in EAC compared to non-malignant esophageal tissue. However, the authors proposed an association between high CD147 expression and advanced disease, for example, lymphatic and distant metastasis, which cannot be confirmed by our data. Our cohort did not reveal any links to any of the assessed clinico-pathological parameters. A lower total number of cases but a rather high fraction of advanced stages (46 of 74 patients staged UICC III/IV) in Huang’s cohort might explain the difference in these findings. In addition, these patients with advanced cancer did not undergo neoadjuvant treatment, which may well have had negatively influenced survival rates independent of CD147 staining (Huang et al. 2015).
However, due to its membranous localization and overexpression, CD147 may prove suitable as a therapeutic target. Previously, CD147 has been suggested as a promising target in several cancers, such as malignant melanoma (Hu et al. 2017) and esophageal cancers (Zhang et al. 2018). CD147 targeting has already been shown to suppress malignant melanoma in vitro and in vivo, highlighting the therapeutic potential of CD147 silencing (Hu et al., 2017). Additionally, overexpression of CD147 has been linked to the development of esophageal cancer and has been considered a potential target for anticancer therapies (Zhang et al. 2018).
The strength of this study is the cohort size for both ESCC and EAC, while published evidence is strongly limited and inconclusive, especially for esophageal adenocarcinoma. The monocentric character and a lost to follow-up ratio of 23.0% and 34.7% are its limitations.
In summary, CD147 expression was detectable in strong intensities in large fractions of our samples but lacked prognostic relevance. However, due to its overexpression, CD147 may represent a promising therapeutic target in a subset of esophageal cancer patients.
p value indicates significance according to the χ2-test. EAC esophageal adenocarcinoma, UICC Union internationale contre le cancer
p value indicates significance according to the χ2-test. p values in bold indicate statistical significance. ESCC esophageal squamous cell cancer, UICC union internationale contre le cancer
Availability of data and material
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Basset P, Bellocq JP, Wolf C, Stoll I, Hutin P, Limacher JM, Chambon P (1990) A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature 348(6303):699–704. https://doi.org/10.1038/348699a0
Biswas C, Zhang Y, De Castro R, Guo H, Nakamura T, Kataoka H, Nabeshima K (1995) The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res 55(2):434–439
Cheng MF, Tzao C, Tsai WC, Lee WH, Chen A, Chiang H, Jin JS (2006) Expression of EMMPRIN and matriptase in esophageal squamous cell carcinoma: correlation with clinicopathological parameters. Dis Esophagus 19(6):482–486. https://doi.org/10.1111/j.1442-2050.2006.00613.x
Dean NR, Newman JR, Helman EE, Zhang W, Safavy S, Weeks DM, Rosenthal EL (2009) Anti-EMMPRIN monoclonal antibody as a novel agent for therapy of head and neck cancer. Clin Cancer Res 15(12):4058–4065. https://doi.org/10.1158/1078-0432.CCR-09-0212
Ellis SM, Nabeshima K, Biswas C (1989) Monoclonal antibody preparation and purification of a tumor cell collagenase-stimulatory factor. Cancer Res 49(12):3385–3391
Feng L, Zhu S, Zhang Y, Li Y, Gong L, Lan M, Zhang W (2013) Expression and clinical significance of HAb18G/CD147 in malignant tumors. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 29(9):958–961
Guo H, Zucker S, Gordon MK, Toole BP, Biswas C (1997) Stimulation of matrix metalloproteinase production by recombinant extracellular matrix metalloproteinase inducer from transfected Chinese hamster ovary cells. J Biol Chem 272(1):24–27
Han ZD, He HC, Bi XC, Qin WJ, Dai QS, Zou J, Zhong WD (2010) Expression and clinical significance of CD147 in genitourinary carcinomas. J Surg Res 160(2):260–267. https://doi.org/10.1016/j.jss.2008.11.838
Hu X, Su J, Zhou Y, Xie X, Peng C, Yuan Z, Chen X (2017) Repressing CD147 is a novel therapeutic strategy for malignant melanoma. Oncotarget 8(15):25806–25813. https://doi.org/10.18632/oncotarget.15709
Huang L, Xu AM, Peng Q (2015) CD147 and MMP-9 expressions in type II/III adenocarcinoma of esophagogastric junction and their clinicopathological significances. Int J Clin Exp Pathol 8(2):1929–1937
Iacono KT, Brown AL, Greene MI, Saouaf SJ (2007) CD147 immunoglobulin superfamily receptor function and role in pathology. Exp Mol Pathol 83(3):283–295. https://doi.org/10.1016/j.yexmp.2007.08.014
Ishibashi Y, Matsumoto T, Niwa M, Suzuki Y, Omura N, Hanyu N, Urashima M (2004) CD147 and matrix metalloproteinase-2 protein expression as significant prognostic factors in esophageal squamous cell carcinoma. Cancer 101(9):1994–2000. https://doi.org/10.1002/cncr.20593
Juhnke M, Heumann A, Chirico V, Hoflmayer D, Menz A, Hinsch A, Luebke AM (2017) Apurinic/apyrimidinic endonuclease 1 (APE1/Ref-1) overexpression is an independent prognostic marker in prostate cancer without TMPRSS2:ERG fusion. Mol Carcinog 56(9):2135–2145. https://doi.org/10.1002/mc.22670
Kanekura T, Chen X, Kanzaki T (2002) Basigin (CD147) is expressed on melanoma cells and induces tumor cell invasion by stimulating production of matrix metalloproteinases by fibroblasts. Int J Cancer 99(4):520–528. https://doi.org/10.1002/ijc.10390
Landras A, Reger de Moura C, Jouenne F, Lebbe C, Menashi S, Mourah S (2019) CD147 is a promising target of tumor progression and a prognostic biomarker. Cancers (basel). https://doi.org/10.3390/cancers11111803
Marieb EA, Zoltan-Jones A, Li R, Misra S, Ghatak S, Cao J, Toole BP (2004) Emmprin promotes anchorage-independent growth in human mammary carcinoma cells by stimulating hyaluronan production. Cancer Res 64(4):1229–1232. https://doi.org/10.1158/0008-5472.can-03-2832
Mirlacher M, Simon R (2010) Recipient block TMA technique. Methods Mol Biol (clifton, NJ) 664:637–644. https://doi.org/10.1007/978-1-60761-806-5_4
Muramatsu T, Miyauchi T (2003) Basigin (CD147): a multifunctional transmembrane protein involved in reproduction, neural function, inflammation and tumor invasion. Histol Histopathol 18(3):981–987. https://doi.org/10.14670/HH-18.981
Nabeshima K, Iwasaki H, Koga K, Hojo H, Suzumiya J, Kikuchi M (2006) Emmprin (basigin/CD147): matrix metalloproteinase modulator and multifunctional cell recognition molecule that plays a critical role in cancer progression. Pathol Int 56(7):359–367. https://doi.org/10.1111/j.1440-1827.2006.01972.x
Peng F, Li H, Ning Z, Yang Z, Li H, Wang Y, Wu Y (2016) CD147 and prostate cancer: a systematic review and meta-analysis. PLoS One 11(9):e0163678. https://doi.org/10.1371/journal.pone.0163678
Sameshima T, Nabeshima K, Toole BP, Yokogami K, Okada Y, Goya T, Wakisaka S (2000) Glioma cell extracellular matrix metalloproteinase inducer (EMMPRIN) (CD147) stimulates production of membrane-type matrix metalloproteinases and activated gelatinase A in co-cultures with brain-derived fibroblasts. Cancer Lett 157(2):177–184. https://doi.org/10.1016/s0304-3835(00)00485-7
Simon R, Mirlacher M, Sauter G (2004) Tissue microarrays. Biotechniques 36(1):98–105. https://doi.org/10.2144/04361RV01
Smyth EC, Lagergren J, Fitzgerald RC, Lordick F, Shah MA, Lagergren P, Cunningham D (2017) Oesophageal cancer. Nat Rev Dis Primers 3:17048. https://doi.org/10.1038/nrdp.2017.48
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Tang Y, Kesavan P, Nakada MT, Yan L (2004) Tumor-stroma interaction: positive feedback regulation of extracellular matrix metalloproteinase inducer (EMMPRIN) expression and matrix metalloproteinase-dependent generation of soluble EMMPRIN. Mol Cancer Res 2(2):73–80
Tang Y, Nakada MT, Kesavan P, McCabe F, Millar H, Rafferty P, Yan L (2005) Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res 65(8):3193–3199. https://doi.org/10.1158/0008-5472.CAN-04-3605
van der Jagt MF, Sweep FC, Waas ET, Hendriks T, Ruers TJ, Merry AH, Span PN (2006) Correlation of reversion-inducing cysteine-rich protein with kazal motifs (RECK) and extracellular matrix metalloproteinase inducer (EMMPRIN), with MMP-2, MMP-9, and survival in colorectal cancer. Cancer Lett 237(2):289–297. https://doi.org/10.1016/j.canlet.2005.06.009
Wan Y, Wu XY (2012) Expression and clinical significance of DAPK1 and CD147 in esophageal squamous cell carcinoma. Zhonghua Zhong Liu Za Zhi 34(1):44–48
Xin X, Zeng X, Gu H, Li M, Tan H, Jin Z, Wang H (2016) CD147/EMMPRIN overexpression and prognosis in cancer: a systematic review and meta-analysis. Sci Rep 6:32804. https://doi.org/10.1038/srep32804
Yan L, Zucker S, Toole BP (2005) Roles of the multifunctional glycoprotein, emmprin (basigin; CD147), in tumour progression. Thromb Haemost 93(2):199–204. https://doi.org/10.1160/TH04-08-0536
Yurchenko V, Constant S, Bukrinsky M (2006) Dealing with the family: CD147 interactions with cyclophilins. Immunology 117(3):301–309. https://doi.org/10.1111/j.1365-2567.2005.02316.x
Zhang T, Li H, Zhang Y, Wang P, Bian H, Chen ZN (2018) Expression of proteins associated with epithelial-mesenchymal transition in esophageal squamous cell carcinoma. Oncol Lett 15(3):3042–3048. https://doi.org/10.3892/ol.2017.7701
Zhu S, Li Y, Mi L, Zhang Y, Zhang L, Gong L, Zhang W (2011) Clinical impact of HAb18G/CD147 expression in esophageal squamous cell carcinoma. Dig Dis Sci 56(12):3569–3576. https://doi.org/10.1007/s10620-011-1812-x
Funding
Open Access funding enabled and organized by Projekt DEAL. This research received no funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Conceptualization: NM, KG. Data curation: NK, J-KG, MT. Formal analysis: NK, J-KG, MR. Investigation: KB, TG, FGU. Supervision: JRI, DP, MR, NM. Writing—original draft: NK, KG, J-KG. Writing—review and editing: KB, TG, FGU, MT, DP, MR, JRI, NM. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
No. WF049/09.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
The authors affirm that participants provided informed consent for publication of the data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Küsters, N., Grupp, K., Grass, JK. et al. CD147 expression lacks prognostic relevance in esophageal cancer. J Cancer Res Clin Oncol 148, 837–844 (2022). https://doi.org/10.1007/s00432-022-03917-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-03917-2