Abstract
Background
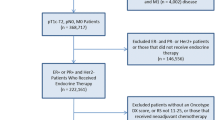
Patient and tumor factors have been associated with rates for pathologic complete response (pCR) to neoadjuvant chemotherapy (NAC) for breast cancer, but variation in pCR rates across facilities has not been studied.
Methods
This study used the National Cancer Data Base to identify women with clinical stages 1–3a breast cancer undergoing NAC from 2010 to 2013. Generalized estimation equation models were used to examine the relationship between facility characteristics and pCR rates, with adjustment for patient and tumor factors, while accounting for patient clustering at facilities. Analyses were stratified by tumor molecular subtype.
Results
Overall, 16,885 women underwent NAC, of whom 3130 (18.5%) were hormone receptor-positive (HR+) and human epidermal growth factor 2-positive (HER2+), 7045 (41.7%) were HR+HER2−, 1847 (10.9%) were HR−HER2+, and 4863 (28.8%) were HR−HER2−. Overall, 4002 of the patients (23.7%) achieved a pCR. The pCR rates were 29.5% for HR+HER2+, 10.8% for HR+HER2−, 45.3% for HR−HER2+, and 30.5% for HR−HER2− tumors. Multivariable analysis showed that pCR rates were significantly higher at high-volume facilities (>75th vs. <25th percentile) for all tumor subtypes except HR+HER2− tumors. Facility location and type were not significant. Adjustment for time from NAC to surgery decreased the likelihood of a pCR in high- versus low-volume facilities, but facility volume remained significantly associated with pCR.
Conclusion
Facility volume, not location or type, was significantly associated with higher pCR rates in this exploratory analysis. Time to surgery has a modest impact on pCR rates across facilities, but further study to identify other potentially modifiable factors is needed.


Similar content being viewed by others
References
Park JW, Liu MC, Yee D, et al. Adaptive Randomization of neratinib in early breast cancer. N Engl J Med. 2016;375:11–22.
Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet London Engl. 2014;384:164–72.
von Minckwitz G, Untch M, Blohmer J-U, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–804.
Kaufmann M, Hortobagyi GN, Goldhirsch A, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: an update. J Clin Oncol. 2006;24:1940–49.
Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460.
U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER), 2014. Retrieved 13 February at http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm305501.pdf.
Loibl S, von Minckwitz G, Untch M, et al. Predictive factors for response to neoadjuvant therapy in breast cancer. Oncol Res Treat. 2014;37:563–68.
Killelea BK, Yang VQ, Wang S-Y, et al. Racial differences in the use and outcome of neoadjuvant chemotherapy for breast cancer: results from the National Cancer Data Base. J Clin Oncol Off J Am Soc Clin Oncol. 2015;33:4267–76.
Vrijens F, Stordeur S, Beirens K, et al. Effect of hospital volume on processes of care and 5-year survival after breast cancer: a population-based study on 25,000 women. Breast Edinb Scotland. 2012;21:261–66.
Hébert-Croteau N, Brisson J, Lemaire J, et al. Investigating the correlation between hospital of primary treatment and the survival of women with breast cancer. Cancer. 2005;104:1343–48.
Pezzin LE, Laud P, Yen TWF, et al. Reexamining the relationship of breast cancer hospital and surgical volume to mortality: an instrumental variable analysis. Med Care. 2015;53:1033–39.
McDermott AM, Wall DM, Waters PS, et al. Surgeon and breast unit volume-outcome relationships in breast cancer surgery and treatment. Ann Surg. 2013;258:808–14.
Albornoz CR, Cordeiro PG, Hishon L, et al. A nationwide analysis of the relationship between hospital volume and outcome for autologous breast reconstruction. Plast Reconstr Surg. 2013;132:192e–200e.
Gooiker GA, van Gijn W, Post PN, et al. A systematic review and meta-analysis of the volume-outcome relationship in the surgical treatment of breast cancer: are breast cancer patients better off with a high-volume provider? Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2010;36(Suppl 1):S27–35.
Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–90.
Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–19.
Stephen Edge, Dvaid R. Byrd, Carolyn C. Compton, et al. AJCC Cancer Staging Manual. 7th ed. Springer-Verlag, New York, 2010.
StataCorp. Stata Statistical Software: Release 14. StataCorp LP, College Station, TX, 2015.
Birkmeyer JD, Siewers AE, Finlayson EVA, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–37.
Ross JS, Normand S-LT, Wang Y, et al. Hospital volume and 30-day mortality for three common medical conditions. N Engl J Med. 2010;362:1110–18.
Reames BN, Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and operative mortality in the modern era. Ann Surg. 2014;260:244–51.
Scharl A, Göhring U-J. Does center volume correlate with survival from breast cancer? Breast Care. 2009;4:237–44.
Nattinger AB, Laud PW, Sparapani RA, et al. Exploring the surgeon volume outcome relationship among women with breast cancer. Arch Intern Med. 2007;167:1958–63.
Kong AL, Pezzin LE, Nattinger AB. Identifying patterns of breast cancer care provided at high-volume hospitals: a classification and regression tree analysis. Breast Cancer Res Treat. 2015;153:689–98.
Vernooij F, Witteveen PO, Verweij E, et al. The impact of hospital type on the efficacy of chemotherapy treatment in ovarian cancer patients. Gynecol Oncol. 2009;115:343–48.
Giri S, Pathak R, Aryal MR, et al. Impact of hospital volume on outcomes of patients undergoing chemotherapy for acute myeloid leukemia: a matched-cohort study. Blood. 2015;125:3359–60.
Hillner BE, Smith TJ, Desch CE. Hospital and physician volume or specialization and outcomes in cancer treatment: importance in quality of cancer care. J Clin Oncol Off J Am Soc Clin Oncol. 2000;18:2327–40.
Yen TWF, Pezzin LE, Li J, et al. Effect of hospital volume on processes of breast cancer care: a National Cancer Data Base study. Cancer. 2016.
Anonymous, 2016. Retrieved 15 February 2017 at https://www.facs.org/~/media/files/quality %20programs/cancer/coc/2016%20coc %20standards %20manual_interactive %20pdf.ashx.
Birkmeyer NJO, Goodney PP, Stukel TA, et al. Do cancer centers designated by the National Cancer Institute have better surgical outcomes? Cancer. 2005;103:435–41.
Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century (Internet). National Academies Press, Washington DC, 2001. Retrieved 13 Febryar 2017 at http://www.ncbi.nlm.nih.gov/books/NBK222274/.
Disclosure
There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ajmani, G.S., James, T.A., Kantor, O. et al. The Impact of Facility Volume on Rates of Pathologic Complete Response to Neoadjuvant Chemotherapy Used in Breast Cancer. Ann Surg Oncol 24, 3157–3166 (2017). https://doi.org/10.1245/s10434-017-5969-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-017-5969-1