Abstract
Background
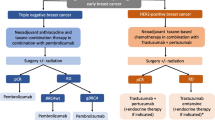
Use of targeted therapy for human epidermal growth factor receptor-2 (HER2)-positive breast cancer has led to improvements in survival. Furthermore, neoadjuvant chemotherapy (NAC) with dual HER2 agents demonstrated improved pathological complete response (pCR) rates. With these data, and with US FDA approval in September 2013 of pertuzumab in the neoadjuvant setting, we hypothesized that the use of NAC for early-stage HER2-positive patients is increasing.
Methods
With Institutional Review Board approval, we reviewed 267 patients with 268 clinical T1 and T2 HER2-positive tumors treated from October 2008 to September 2014. We compared treatment in the early (October 2008–September 2013) to recent (October 2013–September 2014) periods. Statistical analysis was performed using Chi square tests.
Results
Mean patient age was 59 years. Clinical T stage included 6 (2 %) T1mic, 11 (4 %) T1a, 41 (15 %) T1b, 95 (35 %) T1c, and 115 (43 %) T2. Targeted therapy included combinations of trastuzumab, lapatinib, pertuzumab, and neratinib. NAC use increased from 53/219 (24.2 %) in the early group to 19/49 (38.8 %) in the recent group (p = 0.04). Forty-two percent (8/19) of patients in the recent group received neoadjuvant pertuzumab versus 0/53 in the early group (p < 0.0001). More clinically node-negative (cN0) patients received NAC in the recent (12/41, 29.3 %) versus early (20/167, 12.0 %) period (p = 0.01). For T1 tumors, the use of NAC more than doubled between the two time periods (5.6–17.2 %; p = 0.06), while NAC use increased from 48 to 70 % for T2 tumors (p = 0.08). The overall pCR rate after NAC was 48.6 % (35/72).
Conclusions
NAC for HER2-positive breast cancer patients is increasing. Most striking was a substantial increase in NAC for patients with T1 tumors and cN0 disease.

Similar content being viewed by others
References
Goldenberg MM. Trastuzumab, a recombinant DNA-derived humanized monoclonal antibody, a novel agent for the treatment of metastatic breast cancer. Clin Ther. 1999;21(2):309–18.
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–92.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines®): breast cancer. Fort Washington: National Comprehensive Cancer Network; 2015.
Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32.
Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol. 2013;24(9):2278–84.
Siegel RL, Miller KD, Jemal A. Cancer statistics 2015. CA Cancer J Clin. 2015;65(1):5–29.
US FDA. FDA approves Perjeta for neoadjuvant breast cancer treatment. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm370393.htm. Accessed 10 Apr 2015.
American Joint Committee on Cancer (2010) Breast cancer staging. Cancer staging manual 7. American Joint Committee on Cancer, New York.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2012;365(9472):1687–717.
McGale P, Taylor C, Correa C, Cutter D, Duane F, Ewertz M, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127–35.
Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from national surgical adjuvant breast and bowel project B-18. J Clin Oncol. 1997;15(7):2483–93.
Bear HD, Anderson S, Smith RE, Geyer CE, Mamounas EP, Fisher B, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: national surgical adjuvant breast and bowel project protocol B-27. J Clin Oncol. 2006;24(13):2019–27.
Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, De Azambuja E, Aura C, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379(9816):633–40.
Von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–804.
Untch M, Loibl S, Bischoff J, Eidtmann H, Kaufmann M, Blohmer JU, et al. Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. Lancet Oncol. 2012;13(2):135–44.
Perez EA, Romond EH, Suman VJ, Jeong JH, Davidson NE, Geyer CE, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29(25):3366–73.
Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, De Azambuja E, Procter M, Suter TM, et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet. 2013;382(9897):1021–8.
Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–83.
Gonzalez-Angulo AM, Litton JK, Broglio KR, Meric-Bernstam F, Rakkhit R, Cardoso F, et al. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol. 2009;27(34):5700–6.
Fehrenbacher L, Capra A, Quesenberry Jr C, Fulton R, Shiraz P, Habel L. Distant invasive breast cancer recurrence risk in human epidermal growth factor receptor 2-positive T1a and T1b node-negative localized breast cancer diagnosed from 2000 to 2006: a cohort from an integrated health care delivery system. J Clin Oncol. 2014;32(20):2151–8.
Vaz-Luis I, Ottesen RA, Hughes ME, Mamet R, Burstein HJ, Edge SB, et al. Outcomes by tumor subtype and treatment pattern in women with small, node-negative breast cancer: a multi-institutional study. J Clin Oncol. 2014;32(20):2142–50.
Disclosure
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Hilli, Z., Boughey, J.C., Hoskin, T.L. et al. Increasing Use of Neoadjuvant Treatment for T1 and T2 HER2-Positive Tumors. Ann Surg Oncol 22, 3369–3375 (2015). https://doi.org/10.1245/s10434-015-4718-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-015-4718-6