Abstract
Purpose
Agents targeting the human epidermal growth factor receptor 2 (HER2) have improved outcomes of advanced HER2-positive breast cancer with durable responses. We evaluated first-line therapy long-term outcomes in patients responding for more than 1 year.
Methods
We retrospectively identified patients on first-line anti-HER2 therapy at The Royal Marsden Hospital for at least 1 year from 2001 to 2016. Demographics, disease characteristics, treatments and adverse events were recorded. Simple statistics, Fisher’s, Chi squared and log-rank tests were used.
Results
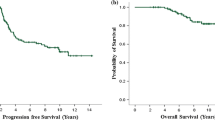
208 patients on treatment for at least 1 year had a median age of 54 years (31–88). 38.0% had de novo metastatic disease and 55.9% were ER positive. Of the relapsed cases, 54.4% previously had trastuzumab. At the time of presentation of metastatic disease, 27.4% of the entire cohort had pulmonary, 43.7% liver and 10.6% brain involvement. 97.1% received trastuzumab and 1.44% lapatinib; 33.2% pertuzumab and trastuzumab. 82.7% received chemotherapy (usually taxanes). 47.6% received maintenance endocrine therapy. Median progression-free survival was 39.5 months and overall survival 81.0 months. Overall response rate was 87.5%. Cardiotoxicity occurred in 4.8% of cases. Seven patients stopped treatment electively after 17–87 months and, so far, all remain in complete remission.
Conclusions
First-line anti-HER2 treatment is associated with median overall survival longer than 6 years in half of the patients free from disease progression after a year, but most still relapse eventually. Response prediction would be key to inform trial design and treatment decisions in this setting.


Similar content being viewed by others
References
Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH (2010) Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol 28(1):92–98
Chia SK, Speers CH, D’Yachkova Y, Kang A, Malfair-Taylor S, Barnett J et al (2007) The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer 110(5):973–979
Ponde N, Brandao M, El-Hachem G, Werbrouck E, Piccart M (2018) Treatment of advanced HER2-positive breast cancer: 2018 and beyond. Cancer Treat Rev 67:10–20
Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M et al (2015) Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 372(8):724–734
Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH et al (2012) Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 366(2):109–119
Gullo G, Zuradelli M, Sclafani F, Santoro A, Crown J (2012) Durable complete response following chemotherapy and trastuzumab for metastatic HER2-positive breast cancer. Ann Oncol 23(8):2204–2205
Witzel I, Muller V, Abenhardt W, Kaufmann M, Schoenegg W, Schneeweis A et al (2014) Long-term tumor remission under trastuzumab treatment for HER2 positive metastatic breast cancer—results from the HER-OS patient registry. BMC Cancer 14:806
Yeo B, Kotsori K, Mohammed K, Walsh G, Smith IE (2015) Long-term outcome of HER2 positive metastatic breast cancer patients treated with first-line trastuzumab. Breast 24(6):751–757
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S et al (2010) American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28(16):2784–2795
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH et al (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31(31):3997–4013
Food and Drug Administration (2007) Guidance for Industry Clinical Trial endpoints for the approval of cancer drugs and biologics
Earl HM, Hiller L, Vallier AL, Loi S, McAdam K, Hughes-Davies L et al (2019) 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet 393:2599–2612
Pivot X, Romieu G, Debled M, Pierga JY, Kerbrat P, Bachelot T et al (2013) 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol 14(8):741–748
Mavroudis D, Saloustros E, Malamos N, Kakolyris S, Boukovinas I, Papakotoulas P et al (2015) Six versus 12 months of adjuvant trastuzumab in combination with dose-dense chemotherapy for women with HER2-positive breast cancer: a multicenter randomized study by the Hellenic Oncology Research Group (HORG). Ann Oncol 26(7):1333–1340
Durkee BY, Qian Y, Pollom EL, King MT, Dudley SA, Shaffer JL et al (2016) Cost-effectiveness of Pertuzumab in human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol 34(9):902–909
Keefe DL (2002) Trastuzumab-associated cardiotoxicity. Cancer 95(7):1592–1600
Henry ML, Niu J, Zhang N, Giordano SH, Chavez-MacGregor M (2018) Cardiotoxicity and cardiac monitoring among chemotherapy-treated breast cancer patients. JACC Cardiovasc Imaging 11(8):1084–1093
Soto-Perez-De-Celis E, Loh KP, Baldini C, Battisti NML, Chavarri-Guerra Y, De Glas NA et al (2018) Targeted agents for HER2-positive breast cancer in older adults: current and future perspectives. Expert Opin Investig Drugs 27(10):787–801
Moja L, Tagliabue L, Balduzzi S, Parmelli E, Pistotti V, Guarneri V et al (2012) Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD006243.pub2/abstract
Dang C, Guo H, Najita J, Yardley D, Marcom K, Albain K et al (2016) Cardiac outcomes of patients receiving adjuvant weekly Paclitaxel and Trastuzumab for node-negative, ERBB2-positive breast cancer. JAMA Oncol 2(1):29–36
Pernas S, Tolaney SM (2019) HER2-positive breast cancer: new therapeutic frontiers and overcoming resistance. Ther Adv Med Oncol 11:1758835919833519
Rugo HS, Im S-A, Wright GLS, Escriva-de-Romani S, DeLaurentiis M, Cortes J et al (2019) SOPHIA primary analysis: a phase 3 (P3) study of margetuximab (M) + chemotherapy (C) versus trastuzumab (T) + C in patients (pts) with HER2 + metastatic (met) breast cancer (MBC) after prior anti-HER2 therapies (Tx). J Clin Oncol 37(15_suppl):1000
Acknowledgements
The authors wish to acknowledge the support of The Cridlan Trust and The Royal Marsden NIHR Biomedical Research Centre for Cancer.
Funding
No funding was required for this retrospective study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr Battisti has received travel grants from Genomic Health and speaker fees from Pfizer. Prof Smith attended an Advisory Board for Roche in 2014. Dr Ring has received advisory board fees from Roche, Novartis, Pfizer and Lilly and speaker fees from Novartis and Pfizer. Dr Tong has no conflict of interest.
Ethical approval
This research project has been reviewed and approved by The Committee for Clinical Review of The Royal Marsden NHS Foundation Trust. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent for systemic anticancer treatment was obtained from all individual participants included in this retrospective analysis.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Battisti, N.M.L., Tong, D., Ring, A. et al. Long-term outcome with targeted therapy in advanced/metastatic HER2-positive breast cancer: The Royal Marsden experience. Breast Cancer Res Treat 178, 401–408 (2019). https://doi.org/10.1007/s10549-019-05406-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05406-6