Abstract
Background
To evaluate the prognostic meaning of lymph node micrometastases in breast cancer patients.
Methods
Between January 2000 and January 2003, 1411 patients with a cT1-2N0 invasive breast carcinoma underwent surgery in 7 hospitals in the Netherlands. Sentinel lymph node biopsy was done in all patients. Based on lymph node status, patients were divided into 4 groups: pN0 (n = 922), pN1micro (n = 103), pN1a (n = 285), and pN≥1b (n = 101). Median follow-up was 6.4 years.
Results
At the end of follow-up, 1121 women were still alive (79.4%), 184 had died (13.0%), and 106 were lost to follow-up (7.5%). Breast cancer recurred in 244 patients: distant metastasis (n = 165), locoregional relapse (n = 83), and contralateral breast cancer (n = 44). Following adjustment for possible confounding characteristics and for adjuvant systemic treatment, overall survival (OS) remained comparable for pN0 and pN1micro and was significantly worse for pN1a and pN≥1b (hazard ratio [HR] 1.18; 95% confidence interval [95% CI] 0.58–2.39, HR 2.47; 95% CI 1.69–3.63, HR 4.36; 95% CI 2.70–7.04, respectively). Disease-free survival (DFS) was similar too in the pN0 and pN1micro group, and worse for pN1a and pN≥1b (HR 0.96; 95% CI 0.56–1.67 vs HR 1.64; 95% CI 1.19–2.27, HR 2.95; CI 1.98–4.42). The distant metastases rate also did not differ significantly between the pN0 and pN1micro group and was worse for pN1a and pN≥1b (HR 1.22; 95% CI 0.60–2.49, HR 2.26; 95% CI 1.49–3.40, HR 3.49; CI 2.12–5.77).
Conclusions
In breast cancer patients survival is not affected by the presence of micrometastatic lymph node involvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Sentinel lymph node biopsy (SLNB) has replaced axillary lymph node dissection (ALND) for lymph node staging in breast cancer. Because of the more extensive pathology examination of sentinel lymph nodes (SLNs), small metastases are observed more frequently.1–7 Lymph node micrometastases (LNMM) or isolated tumor cells are observed in up to 23% of breast cancer patients.1,6,7
Recent studies have refueled the discussion regarding the prognostic meaning of LNMM.8–11 Some authors argue that LNMM do influence survival adversely, while others observed comparable survival for patients with micrometastases and patients without metastatic lymph node involvement. Although the discussion started well before the introduction of the SLNB, SLNB data are conflicting too. In a previous study we observed similar overall survival (OS) for patients classified as pN0 and pN1micro.12,13 The main weaknesses of that study were a limited follow-up period and the single-institutional experience.10
We therefore now report again on the prognostic meaning of micrometastatic lymph node involvement in a large cohort of consecutive patients from 7 Dutch hospitals with long-term follow-up.
Patients and Methods
Study Cohort
Data were collected on all patients who underwent surgery including SLNB between January 2000 and January 2003 for cT1–2N0 primary invasive breast cancer in 7 hospitals in the Netherlands: University Medical Center Utrecht, Diakonessen Hospital Utrecht, St Antonius Hospital Utrecht, Amphia Hospital Breda, Jeroen Bosch Hospital’s Hertogenbosch, Reinier de Graaf Hospital Delft, and Canisius Hospital Nijmegen. During the study period 1584 patients underwent surgical treatment. To avoid the difficulty of ascribing patient outcome to tumor-specific lymph node status, a number of cases were excluded: previous history of cancer (n = 84), synchronous contralateral breast cancer (n = 33), or multifocal carcinoma (n = 56). The cohort available for analysis consisted of 1411 patients.
SLNB and Pathology Examination of Lymph Nodes and the Primary Tumor
To visualize and identify SLNs, a preoperative lymphoscintigraphy was done, while a patent blue dye injection (Bleu patenté V, Laboratoire Guerbet, Aulnay-sous-Bois, France) and a γ-ray detection probe were used intraoperatively. The definitive pathology examination of SLNs was done according to the Dutch national guidelines: SLNs were formalin-fixed and paraffin-embedded, and starting from the center at least 3 cuts from both halves were taken at 250-μm intervals.14. The sections were stained both with hematoxylin and eosine (H&E) and immunohistochemically (IHC) with an antibody against keratin. When the axillary SLN contained micrometastases or macrometastases, patients were advised to undergo an ALND. Lymph nodes retrieved by ALND were examined by examining 1 central cut from every lymph node.
In addition, the diameter, malignancy grade, and estrogen (ER) and progesterone (PR) receptor status of the primary tumor were assessed. At the time of the study, HER2/neu status was not routinely examined. There were institutional differences in the classification of malignancy grade. In 4 hospitals the modified Bloom and Richardson (BR) grade was assessed, while the mitotic activity index (MAI) was preferably used to assess tumor aggressiveness in the other hospitals.15 Three hospitals used both methods.
Classification of Metastatic Lymph Node Involvement
Lymph node involvement was categorized according to the 6th edition of the UICC-TNM classification: pN0, no lymphogenic metastasis; pNitc, isolated tumor cells (<0.2 mm); pN1micro, regional lymphogenic metastasis with a size between 0.2 and 2.0 mm; pN1a, 1–3 axillary lymphogenic macrometastases (at least one >2.0 mm); pN1b, 1 positive internal mammary node (>2.0 mm); pN1c, a combination of pN1a and pN1b; pN2, 4–9 ipsilateral axillary lymphogenic macrometastases; pN3, more than 9 axillary lymphogenic macrometastases.16 Original pathology reports were adhered to for the classification of metastatic lymph node involvement. When information regarding the size of micrometastases was missing, original slides were reviewed.
Postsurgical Treatment
According to the Dutch national guideline, adjuvant systemic and/or radiotherapeutic treatment was based on axillary lymph node status and primary tumor characteristics.14 In patients classified as pN1micro, the national guideline did not advise adjuvant systemic therapy on a routine basis. Acknowledging that the prognostic meaning of LNMM was unclear, this was routinely discussed with the patients and the choice to give hormonal therapy and/or chemotherapy was made by the physician and the patient.
Follow-Up
Follow-up started at the date of first operation. Patients were seen twice yearly during outpatient visits. Follow-up was concluded between April and November 2008. Dates of locoregional recurrence, contralateral breast cancer, bone or visceral metastases, and death were recorded.
Analysis
The main focus of our study was to compare pTNM pN1micro with pN0. We categorized patients into 4 groups on the basis of metastases in regional lymph nodes: pN0 (including pNitc, n = 23), pN1micro, pN1a, and pN≥1b. The following outcome measures were defined: overall survival (OS), disease-free survival (DFS), and its individual components: locoregional recurrence, contralateral breast cancer, and distant metastases (distinguishing between bone and visceral). Covariables were hospital, age at operation, tumor size, BR grade, MAI, ER/PR status, HER2/neu status, and adjuvant radiotherapy, hormonal therapy, or chemotherapy.
The relation between lymph node status and patient outcome was analyzed using Cox proportional hazards regression. Several models were made for each outcome of interest: with adjustment for hospital (adjusted model 1), with additional adjustment for age (continuous), tumor size (continuous), and BR grade (adjusted model 2), and with additional adjustment for adjuvant treatment (adjusted model 3). Other covariables were not included in the models because of their modest baseline differences (ER and PR status) and the large number of missing values (MAI and HER2/neu), as well as to keep the ratio of variables to the number of outcome events in the models within reasonable boundaries.
Missing Data and Exploration of Heterogeneity
Not all patients had complete records for all covariables, mostly due to institutional differences in expressing malignancy grade and the fact that HER2/neu status was not assessed routinely before 2004. Missing values included: tumor size (n = 8), BR grade (n = 350), MAI (n = 505), ER status (n = 41), PR status (n = 55), HER2/neu status (n = 1215), adjuvant radiotherapy (n = 3), adjuvant hormonal therapy (n = 12), and adjuvant chemotherapy (n = 13). Missing values were imputed for all variables except HER2/neu and MAI, which were consequently not included in multivariable analyses.
As the data were obtained from different hospitals, we explored heterogeneity in the proportion of patients with pN1micro and heterogeneity in overall DFS and OS risk between the hospitals. Also, we assessed heterogeneity across hospitals in the association between nodal status and DFS, but not OS as the number of deaths in each hospital was too small.
A more detailed description of the methods can be found in the Supplementary Appendix.
Results
The median age of the 1411 patients was 57 years. Overall, 922 women (65.3%) were classified as pN0, 103 (7.3%) as pN1micro, 285 (20.2%) as pN1a, and 101 (7.2%) as pN≥1b. Baseline characteristics, in relation to lymph node status, are shown in Table 1. Notably, women with higher nodal status were more likely to be younger and had larger and less well-differentiated primary tumors. No clear differences were observed for MAI, ER/PR and HER2/neu. The frequency of patients with pN1micro disease varied significantly between each hospital with a median of 6.3% (range 4.2%–12.1%) per hospital (P = .006).
At the end of follow-up, with a median of 6.4 years and totalling 8676 years of observation, 1121 women were still alive (79.4%), 184 had died (13.0%), and 106 were lost to follow-up (7.5%). Also, 123 patients died with and 61 patients died without breast cancer recurrence, the overall death rate was 2.1% per year. Breast cancer recurred in 244 patients: distant metastasis (n = 165), locoregional relapse (n = 83), and contralateral breast cancer (n = 44). The annual incidence rates were 1.0% for locoregional recurrence, 0.5% for contralateral cancer, and 2.0% for metastases (visceral: 1.5%; bone: 1.3%). Taking differences in baseline characteristics into account, overall OS and DFS were not different between hospitals (P = .49 and P = .21, respectively).
Adjuvant Postoperative Treatment
Adjuvant hormonal therapy and chemotherapy were given more frequently with increasing nodal status (Table 2). In the pN1micro group, 41% received chemotherapy and 63% hormonal therapy (20% received both).
Patient Outcome
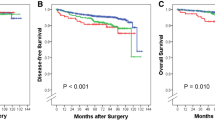
After adjustment for hospital and differences in age, tumor size, and BR grade, OS in the pN1micro group was comparable to OS of patients with pN0 disease (hazard ratio [HR] 0.99; 95% confidence interval [CI]: 0.49–1.98). Compared with pN0 disease, OS was gradually worse for pN1a and pN≥1b patients (HR 1.99, 95% CI: 1.41–2.82 and HR 3.42; 95% CI: 2.21–5.30, respectively; Table 3 and Fig. 1a). Further adjustment for adjuvant therapy increased the HR for pN1a and pN≥1b, but did not substantially change the pN1micro risk estimate (Table 3).
a Overall survival for clinically T1-2 breast cancer patients according to lymph node status. Median follow-up 6.4 years, on the basis of Cox proportional hazard analyses adjusted for age, age2, tumor size, tumor size2, BR-grade, lymph node status and adjuvant treatment. b Disease free survival for clinically T1-2 breast cancer patients according to lymph node status. Median follow-up 6.4 years, on the basis of Cox proportional hazard analyses adjusted for age, age2, tumor size, BR-grade, lymph node status and adjuvant treatment
Similarly, after adjustment for hospital, age, tumor size, and BR grade, DFS was not statistically different in the pN1micro group compared with pN0 patients (HR: 0.84; 95% CI: 0.49–1.43) and was significantly worse for the pN1a or pN≥1b patients (HR: 1.35; 95% CI: 1.02–1.79 and HR: 2.29, 95% CI: 1.60–3.29, respectively; Table 3 and Fig. 1b). Additional adjustment for adjuvant therapy did not change the HR between the pN1micro and pN0 groups and strengthened the HR for both the pN1a and pN≥1b groups compared with pN0 patients (Table 3).
There was a marginal, nonsignificant, increased risk of developing distant metastases in the pN1micro group compared with pN0 patients (hospital, age, tumor size, and BR grade adjusted; HR: 1.10; 95% CI: 0.55–2.22), in contrast with a substantially increased risk in the pN1a and pN≥1b groups (hospital, age, tumor size, and BR grade adjusted; HR: 2.02; 95% CI: 1.39–2.92 and HR: 3.22; 95% CI: 2.06–5.05, respectively; Table 4).
The association between nodal status and DFS (adjusted for age, tumor size, BR grade, and adjuvant therapy) was not different between the 7 hospitals (P for interaction between hospital and nodal status: 0.74). The homogeneity chi-square P value was .88 for pN1micro HRs between hospitals, .81 for pN1a, and .75 for pN≥1b—all versus pN0—indicating no evidence of heterogeneity between hospitals.
Sensitivity Analysis with Complete-Case Approach
Analyzing the data with a complete-case approach (thus without imputation of missing values) yielded similar results. This approach led on average to marginally stronger relations (on average 1% inflation of risk estimates [standard deviation 18%]).
Discussion
In this multicenter cohort study of breast cancer patients who underwent SLNB, the presence of LNMM did not affect outcome. Patients with LNMM did not have significantly different OS and DFS compared with patients without lymph node metastases, while they had a substantially better prognosis than patients with pN1 disease.
The main strengths of the present study are the long follow-up (close to 6.5 years median) and the multicenter approach, contributing to a study population more reflective of all breast cancer cases compared with our previous single institution study.10 Furthermore, the study cohort is large with more than 100 patients with LNMM, and we performed multivariable analyses to control for potential confounding factors in contrast to the majority of similar studies until now.17
A weakness of this study is its retrospective nature. Pathology procedures were not standardized, leading to differences between hospitals especially with regard to classifying malignancy grade. This led to incomplete information on BR grade and MAI, which was overcome statistically by imputing data. Furthermore, the observed frequency of pN1micro patients differed significantly between the hospitals, and the overall 7.3% was lower than expected. In the aforementioned single-institution study, we observed LNMM in 11.5% of the patients, and others have reported frequencies of LNMM in up to 23% of the patients.1,6,7,10 Assuming that misclassification may have occurred, the impact of such misclassification seems minor, since outcome endpoints for the different N classes showed a similar pattern irrespective of the frequency of LNMM in the hospitals.
Even when adjusting for adjuvant systemic therapy, a similar outcome was observed in patients with pN0 and pN1micro disease, and the outcome was substantially better than the outcome of patients with pN1 disease. As the estimated risk reductions of adjuvant hormonal and chemotherapy (0.66 and 0.77, respectively, for OS) were in line with reported risk reductions of these therapies in randomized clinical trials, this adjustment is adequate.18
The controversy regarding the prognostic meaning of LNMM is not new. Intuitively, it would make sense that limited lymph node involvement has a limited effect on prognosis, as the extent of lymph node involvement is directly related to OS. In older “ALND” studies, when micrometastases were rarely detected, some reported a prognostic impact while others did not.12,13,19 The largest study, based on population-based SEER-data, showed that patients with LNMM had a prognosis that was in between the prognosis of node negative patients and of pN1 patients.20 The January 2010 7th edition of the American Joint Committee on Cancer Staging Manual has revised the staging of LNMM. Traditionally, LNMM was grouped with macrometastases (stage II and above), but is now downstaged to stage IB for small tumors, in order to “indicate the better prognosis for the subset of breast cancer patients and to facilitate further investigation.”21 This revision is based on SEER data, and no reference is made to other studies.20 Some studies based on lymph nodes examined by SLNB suggested a worse DFS in patients with LNMM without a significant effect on OS, but others, including the previous report from one of the institutions participating in the present study, did not find a significant relation between DFS or OS and the presence of LNMM.8,10,11,17
These reports and the present study contrast sharply with the conclusions from the recently published MIRROR study that did observe a significant impact of both micrometastases and isolated tumor cells on outcome, as well as a substantial beneficial effect of adjuvant treatment in these patients.9 Interestingly, the MIRROR study includes patients from all Dutch hospitals and was partly conducted during the same years as the study we currently report on. Consequently, a substantial proportion of the patients from the present study were in the MIRROR study too.
The most important difference between the two studies is the endpoint that was used to draw conclusions. As of now, the MIRROR study has not reported on OS but merely on DFS. In our view, the appropriateness of focusing on DFS is questionable, particularly in patients with a good prognosis due to a low risk of distant metastases. In these patients the contribution of contralateral breast cancer and local recurrence to the composite endpoint DFS is large. In the supplementary appendix accompanying the published version of the MIRROR study (accessible through the NEJM website) supplementary Table 2 lists the 5-year event rates of individual components of DFS. While the reported DFS after 5 years was 86% in the pN0 and 76% in the pN1micro group, respectively, the 5-year rates of distant metastases were 2.8% in the pN0 group and 4.6% in the pN1micro group (without adjuvant treatment). As such, distant metastases were less frequent than contralateral breast cancers and locoregional recurrences and probably also less common than death due to unrelated causes, although these data are not presented. Similarly, the beneficial effects of adjuvant treatment in the pN1micro group on DFS seem to be largely driven by effects on contralateral breast cancer and locoregional recurrence. Based on these observations, we are not convinced that the reported significant DFS difference will translate into an OS difference. Especially since large breast cancer trials have reported inconsistent results for DFS and OS.22
The relevance of the prognostic meaning of micrometastatic disease in breast cancer patients is the answer to the clinical problem of whether pN1micro on its own should be an indication for adjuvant systemic treatment. Indications for adjuvant systemic therapy based on primary tumor characteristics have expanded. Hence, nowadays 80% of the LNMM patients of the present cohort would receive adjuvant systemic therapy based on the primary tumor characteristics. The present data from a large multicenter population with a long-term follow-up do not support the use of adjuvant systemic treatment in patients only because they have LNMM. However, this issue warrants ongoing debate and should be addressed preferably in a randomized trial using overall survival as its primary endpoint.
References
Cserni G. Metastases in axillary sentinel lymph nodes in breast cancer as detected by intensive histopathological work up. J Clin Pathol. 1999;52:922–4.
Cserni G. Axillary staging of breast cancer and the sentinel node. J Clin Pathol. 2000;53:733–41.
Cserni G. Complete sectioning of axillary sentinel nodes in patients with breast cancer. Analysis of two different step sectioning and immunohistochemistry protocols in 246 patients. J Clin Pathol. 2002;55:926–31.
Cserni G, Amendoeira I, Apostolikas N, Bellocq JP, Bianchi S, Bussolati G, et al. Pathological work-up of sentinel lymph nodes in breast cancer. Review of current data to be considered for the formulation of guidelines. Eur J Cancer. 2003;39:1654–67.
Tjan-Heijnen VC, Bult P, de Widt-Evert LM, Ruers TJ, Beex LV. Micro-metastases in axillary lymph nodes: an increasing classification and treatment dilemma in breast cancer due to the introduction of the sentinel lymph node procedure. Breast Cancer Res Treat. 2001;70:81–8.
van der Heiden-van der Loo M, Bezemer PD, Hennipman A, Siesling S, van Diest PJ, Bongers V, et al. Introduction of sentinel node biopsy and stage migration of breast cancer. Eur J Surg Oncol. 2006;32:710–4.
van Rijk MC, Peterse JL, Nieweg OE, Oldenburg HSA, Rutgers EMTh, Kroon, BBR. Additional axillary metastases and stage migration in breast cancer patients with micrometastases or submicrometastases in sentinel lymph nodes. Cancer. 2006;107:467–71.
Colleoni M, Rotmensz N, Peruzzotti G, Maisonneuve P, Mazzarol G, Pruneri G, et al. Size of breast cancer metastases in axillary lymph nodes: clinical relevance of minimal lymph node involvement. J Clin Oncol. 2005;23:1379–89.
de Boer M, van Deurzen CH, van Dijck JA, Borm GF, van Diest PJ, Adang EM, et al. Micrometastases or isolated tumor cells and the outcome of breast cancer. N Engl J Med. 2009;361:653–63.
Gobardhan PD, Elias SG, Madsen EVE, Bongers V, Ruitenberg HJM, Perre CI, et al. Prognostic value of micrometastases in sentinel lymph nodes of patients with breast carcinoma: a cohort study. Ann Oncol. 2009;20:41–8.
Hansen NM, Grube B, Ye X, Turner RR, Brenner RJ, Sim MS, et al. Impact of micrometastases in the sentinel node of patients with invasive breast cancer. J Clin Oncol. 2009;27:4679–84.
Huvos AG, Hutter RV, Berg JW. Significance of axillary macrometastases and micrometastases in mammary cancer. Ann Surg. 1971;173:44–6.
Rosen PP, Saigo PE, Braun DW, Weathers E, Fracchia AA, Kinne DW. Axillary micro- and macrometastases in breast cancer: prognostic significance of tumor size. Ann Surg. 1981;194:585–91.
Rutgers EJ, Nortier JW, Tuut MK, van Tienhoven G, Struikmans H, Bontenbal M, et al. [Dutch Institute for Healthcare Improvement guideline, “Treatment of breast cancer”]. Ned Tijdschr Geneeskd. 2002;146:2144–51.
Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–10.
Wittekind C, Greene FL, Hutter RVP, Klimpfinger M, Sobin LH. Breast tumours. In: TNM Atlas, Illustrated Guide to the TNM/pTNM Classification of Malignant Tumours. New York: Springer; 2005:207–23.
de Boer M, van Dijck JA, Bult P, Borm GF, Tjan-Heijnen VCG. Breast cancer prognosis and occult lymph node metastases, isolated tumor cells, and micrometastases. J Natl Cancer Inst. 2010;102:410–25.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717.
Kuijt GP, Voogd AC, van de Poll-Franse LV, Scheijmans LJEE, van Beek MWPM, Roumen RMH. The prognostic significance of axillary lymph-node micrometastases in breast cancer patients. Eur J Surg Oncol. 2005;31:500–5.
Chen SL, Hoehne FM, Giuliano AE. The prognostic significance of micrometastases in breast cancer: a SEER population-based analysis. Ann Surg Oncol. 2007;14:3378–84.
AJCC. Breast. In: Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, eds. AJCC Cancer Staging Manual. New York: Springer, 2010:347–76.
Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53.
Acknowledgment
S.G.E. is supported by a Fellowship from the Dutch Cancer Society KWF.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Gobardhan, P.D., Elias, S.G., Madsen, E.V.E. et al. Prognostic Value of Lymph Node Micrometastases in Breast Cancer: A Multicenter Cohort Study. Ann Surg Oncol 18, 1657–1664 (2011). https://doi.org/10.1245/s10434-010-1451-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-010-1451-z