Abstract
Background
Carmustine-releasing wafers (Gliadel®) have been available and reimbursed in France since 2005.
Methods
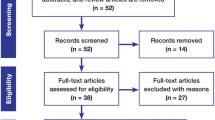
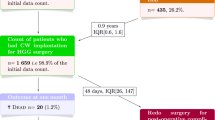
A retrospective multicenter study was conducted in 26 French Departments of Neurosurgery to analyze practices of French neurosurgeons using Gliadel, compare the adverse effects and survival with those of previous phase III trials, and assess survival in patients with newly diagnosed malignant gliomas (MG) receiving Gliadel plus radiochemotherapy with temozolomide (TMZ). A total of 163 patients who received Gliadel for MG were included in this study: 83 (51%) with newly diagnosed MG and 80 (49%) with recurrent MG. In the newly diagnosed group, 51.8% of patients received radiochemotherapy with TMZ.
Results
Adverse events (AEs) emerged in 44.6% of the population, including 6% with septic abscess. The AE rate was not statistically correlated with adjuvant use of TMZ. For the newly diagnosed group, median survival was 17 months. Total or subtotal resection appeared to have a great impact on survival (P = 0.016), as did treatment with adjuvant radiotherapy (P = 0.004).
For the group with recurrent MG, median survival was 7 months. Total or subtotal resection excision appeared to have a great impact on survival (P = 0.002), as did preoperative Karnowsky Scale (PO-KPS) (P = 0.012).
Conclusions
Survival rates for newly diagnosed patients were better than those reported in previous phase III trials. The combination of Gliadel and radiochemotherapy with TMZ was well tolerated and appeared to increase survival without increasing AEs.
Similar content being viewed by others
References
Surawicz TS, et al. Descriptive epidemiology of primary brain and CNS tumors: results from the Central Brain Tumor Registry of the United States, 1990–1994. Neuro Oncol. 1999;1(1):14–25.
Walker MD, et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med. 1980;303(23):1323–9.
Fine HA, et al. Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer. 1993;71(8):2585–97.
Brem H, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas The Polymer-brain Tumor Treatment Group. Lancet. 1995;345(8956):1008–12.
Westphal M, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5(2):79–88.
Westphal M, et al. Gliadel wafer in initial surgery for malignant glioma: long-term follow-up of a multicenter controlled trial. Acta Neurochir (Wien). 2006;148(3):269–75.
Stupp R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96.
LaRocca RV, et al. A phase 2 study of radiation with concomitant and then sequential temozolomide (TMZ) in patients with newly diagnosed supratentorial high-grade malignant glioma who have undergone surgery with carmustine (BCNU) wafer insertion [abstract]. Neuro Oncol. 2006;8(4):445.
Sarkaria JN, et al. Mechanisms of chemoresistance to alkylating agents in malignant glioma. Clin Cancer Res. 2008;14(10):2900–8.
Daumas-Duport C, et al. [Gliomas: WHO and Sainte-Anne Hospital classifications]. Ann Pathol. 2000;20(5):413–28.
Rich JN, et al. Overall survival of primary glioblastoma (GBM) patients receiving carmustine (BCNU) wafers followed by radiation (RT) and concurrent temozolomide (TMZ) plus rotational multi-agent chemotherapy [abstract]. J Clin Oncol. 2007;25:2070.
Lawson HC, et al. Interstitial chemotherapy for malignant gliomas: the Johns Hopkins experience. J Neurooncol. 2007;83(1):61–70.
McGovern PC, et al. Risk factors for postcraniotomy surgical site infection after 1,3-bis (2-chloroethyl)-1-nitrosourea (Gliadel) wafer placement. Clin Infect Dis. 2003;36(6):759–65.
Attenello FJ, et al. Use of Gliadel (BCNU) wafer in the surgical treatment of malignant glioma: a 10-year institutional experience. Ann Surg Oncol. 2008;15(10):2887–93.
Engelhard HH, Tumor bed cyst formation after BCNU wafer implantation: report of two cases. Surg Neurol. 2000;53(3):220–4.
Kleinberg LR, et al. Clinical course and pathologic findings after Gliadel and radiotherapy for newly diagnosed malignant glioma: implications for patient management. Cancer Invest. 2004;22(1):1–9.
McGirt MJ, et al. Management of tumor bed cysts after chemotherapeutic wafer implantation Report of four cases. J Neurosurg. 2002;96(5):941–5.
Kapsalaki EZ, Gotsis ED, Fountas KN. The role of proton magnetic resonance spectroscopy in the diagnosis and categorization of cerebral abscesses. Neurosurg Focus. 2008;24(6):E7.
Lai PH, et al. Brain abscess and necrotic brain tumor: discrimination with proton MR spectroscopy and diffusion-weighted imaging. AJNR Am J Neuroradiol. 2002;23(8):1369–77.
Gururangan S, et al. Phase I study of Gliadel wafers plus temozolomide in adults with recurrent supratentorial high-grade gliomas. Neuro Oncol. 2001;3(4):246–50.
Subach BR, et al. Morbidity and survival after 1,3-bis(2-chloroethyl)-1-nitrosourea wafer implantation for recurrent glioblastoma: a retrospective case-matched cohort series. Neurosurgery. 1999;45(1):17–22.
Weber EL, Goebel EA. Cerebral edema associated with Gliadel wafers: two case studies. Neuro Oncol. 2005;7(1):84–9.
Gallego JM, Barcia JA, Barcia-Marino C. Fatal outcome related to carmustine implants in glioblastoma multiforme. Acta Neurochir (Wien). 2007;149(3):261–5.
Wu MP, et al. In vivo versus in vitro degradation of controlled release polymers for intracranial surgical therapy. J Biomed Mater Res. 1994;28(3):387–95.
Darakchiev BJ, et al., Safety and efficacy of permanent iodine-125 seed implants and carmustine wafers in patients with recurrent glioblastoma multiforme. J Neurosurg. 2008;108(2):236–42.
Limentani SA, et al. A phase I trial of surgery, Gliadel wafer implantation, and immediate postoperative carboplatin in combination with radiation therapy for primary anaplastic astrocytoma or glioblastoma multiforme. J Neurooncol. 2005;72(3):241–4.
Weingart J, et al. Phase I trial of polifeprosan 20 with carmustine implant plus continuous infusion of intravenous O6-benzylguanine in adults with recurrent malignant glioma: new approaches to brain tumor therapy CNS consortium trial. J Clin Oncol. 2007;25(4):399–404.
Vredenburgh JJ, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13(4):1253–9.
Acknowledgment
We would like to thank the neurosurgeons, radiotherapists, oncologists, radiologists, and nurses who participated to this study and provided patient care. We thank also belongs to the authors Karim P. Moubarak MD for editorial assistance. Special thanks to: P Toussaint (Amiens); GA Czorny, J Godard, H Katranji (Besançon); T Faillot, M Kalamarides, G Iakolev (Beaujon-Paris); T Riem, A Rougier, P Monteil, G Penchet, JR Vigne, D Loguoro (Bordeaux); E Emery, JM Derlon, R Gadan, E. Lechapt-Zalcman (Caen); R Giacomelli, A. Monjour, B. Stilhart (Colmar); D Hoffmann (Grenoble); A Desplat (Pau), X Morandi, A Hamlat, L Riffaud, G Brassier (Rennes); O Langlois, F. Proust (Rouen); R Duthel (St Etienne); P François (Tours); M Gigaud, M Tremoulet (Toulouse); L Bauchet (Montpellier); C Pinelli (Nancy); K Moubarak, P Decq, and JP NGuyen (Paris-Henri Mondor).
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Menei, P., Metellus, P., Parot-Schinkel, E. et al. Biodegradable Carmustine Wafers (Gliadel) Alone or in Combination with Chemoradiotherapy: The French Experience. Ann Surg Oncol 17, 1740–1746 (2010). https://doi.org/10.1245/s10434-010-1081-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-010-1081-5