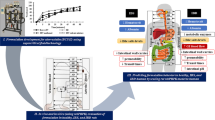
Abstract
The common disorders irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD) can modify the drugs' pharmacokinetics via their induced pathophysiological changes. This work aimed to investigate the impact of these two diseases on pravastatin oral bioavailability. Rat models for IBS and IBD were used to experimentally test the effects of IBS and IBD on pravastatin pharmacokinetics. Then, the observations made in rats were extrapolated to humans using a mechanistic whole-body physiologically-based pharmacokinetic (wbPBPK) model. The rat in vivo studies done herein showed that IBS and IBD decreased serum albumin (> 11% for both), decreased PRV binding in plasma, and increased pravastatin absolute oral bioavailability (0.17 and 0.53 compared to 0.01) which increased plasma, muscle, and liver exposure. However, the wbPBPK model predicted muscle concentration was much lower than the pravastatin toxicity thresholds for myotoxicity and rhabdomyolysis. Overall, IBS and IBD can significantly increase pravastatin oral bioavailability which can be due to a combination of increased pravastatin intestinal permeability and decreased pravastatin gastric degradation resulting in higher exposure. This is the first study in the literature investigating the effects of IBS and IBD on pravastatin pharmacokinetics. The high interpatient variability in pravastatin concentrations as induced by IBD and IBS can be reduced by oral administration of pravastatin using enteric-coated tablets. Such disease (IBS and IBD)-drug interaction can have more drastic consequences for narrow therapeutic index drugs prone to gastric degradation, especially for drugs with low intestinal permeability.
Graphical Abstract




Similar content being viewed by others
References
Farnier M, Davignon J. Current and future treatment of hyperlipidemia: the role of statins. Am J Cardiol. 1998;82:3J-10J. https://doi.org/10.1016/S0002-9149(98)00423-8.
Bristol-Myers Squibb Company. Pravachol (Pravastatin Sodium) 2011. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/019898Orig1s061.pdf. Accessed 19 Nov 2023.
Mao J, Doshi U, Wright M, Hop CE, Li AP, Chen Y. Prediction of the pharmacokinetics of pravastatin as an OATP substrate using plateable human hepatocytes with human plasma data and PBPK modeling. CPT Pharmacometrics Syst Pharmacol. 2018;7:251–8. https://doi.org/10.1002/psp4.12283.
Pan H. Clinical pharmacology of pravastatin, a selective inhibitor of HMG-CoA reductase. Eur J Clin Pharmacol. 1991;40:S15–8. https://doi.org/10.1007/BF03216282.
Singhvi S, Pan H, Morrison R, Willard D. Disposition of pravastatin sodium, a tissue-selective HMG-CoA reductase inhibitor, in healthy subjects. Br J Clin Pharmacol. 1990;29:239–43. https://doi.org/10.1111/j.1365-2125.1990.tb03626.x.
Ruiz-Picazo A, Colón-Useche S, Perez-Amorós B, González-Álvarez M, Molina-Martínez I, González-Álvarez I, et al. Investigation to explain bioequivalence failure in pravastatin immediate-release products. Pharmaceutics. 2019;11:663. https://doi.org/10.3390/pharmaceutics11120663.
Lee JB, Zgair A, Taha DA, Zang X, Kagan L, Kim TH, et al. Quantitative analysis of lab-to-lab variability in Caco-2 permeability assays. Eur J Pharm Biopharm. 2017;114:38–42. https://doi.org/10.1016/j.ejpb.2016.12.027.
Brain-Isasi S, Requena C, Álvarez-Lueje A. Stability study of pravastatin under hydrolytic conditions assessed by HPLC. J Chil Chem Soc. 2008;53:1684–8. https://doi.org/10.4067/S0717-97072008000400010.
Mehanna MM, Shabarek MI, Elmartadny HA. Spray-dried pH-sensitive microparticles: effectual methodology to ameliorate the bioavailability of acid labile pravastatin. Drug Dev Ind Pharm. 2019;45:485–97. https://doi.org/10.1080/03639045.2018.1562465.
Triscari J, O’Donnell D, Zinny M, Pan HY. Gastrointestinal absorption of pravastatin in healthy subjects. J Clin Pharmacol. 1995;35:142–4. https://doi.org/10.1002/j.1552-4604.1995.tb05002.x.
Li S, Fei G, Fang X, Yang X, Sun X, Qian J, et al. Changes in enteric neurons of small intestine in a rat model of irritable bowel syndrome with diarrhea. J Neurogastroenterol Motil. 2016;22:310–20. https://doi.org/10.5056/jnm15082.
Wojtal KA, Eloranta JJ, Hruz P, Gutmann H, Drewe J, Staumann A, et al. Changes in mRNA expression levels of solute carrier transporters in inflammatory bowel disease patients. Drug Metab Dispos. 2009;37:1871–7. https://doi.org/10.1124/dmd.109.027367.
Katinios G, Casado-Bedmar M, Walter SA, Vicario M, González-Castro AM, Bednarska O, et al. Increased colonic epithelial permeability and mucosal eosinophilia in ulcerative colitis in remission compared with irritable bowel syndrome and health. Inflamm Bowel Dis. 2020;26:974–84. https://doi.org/10.1093/ibd/izz328.
Lee H, Park JH, Park DI, Kim HJ, Cho YK, Sohn CI, et al. Mucosal mast cell count is associated with intestinal permeability in patients with diarrhea predominant irritable bowel syndrome. J Neurogastroenterol Motil. 2013;19:224. https://doi.org/10.5056/jnm.2013.19.2.244.
Bai JP, Burckart GJ, Mulberg AE. Literature review of gastrointestinal physiology in the elderly, in pediatric patients, and in patients with gastrointestinal diseases. J Pharm Sci. 2016;105:476–83. https://doi.org/10.1002/jps.24696.
Shao Y-Y, Huang J, Ma Y-R, Han M, Ma K, Qin H-Y, et al. Serum serotonin reduced the expression of hepatic transporter Mrp2 and P-gp via regulating nuclear receptor CAR in PI-IBS rats. Can J Physiol Pharmacol. 2015;93:633–9. https://doi.org/10.1139/cjpp-2015-0039.
Drozdzik M, Czekawy I, Oswald S, Drozdzik A. Intestinal drug transporters in pathological states: An overview. Pharmacol Rep. 2020;72:1173–94. https://doi.org/10.1007/s43440-020-00139-6.
Shekhar R, Biyyani RS, Putka BS, Mullen KD. Dyslipidemia and lipoprotein profiles in patients with inflammatory bowel disease. J Clin Lipidol. 2010;4:478–82. https://doi.org/10.1016/j.jacl.2010.08.021.
Fiderkiewicz B, Rydzewska-Rosołowska A, Myśliwiec M, Birecka M, Kaczanowska B, Rydzewska G, et al. Factors associated with irritable bowel syndrome symptoms in hemodialysis patients. World J Gastroenterol. 2011;17:1976. https://doi.org/10.3748/wjg.v17.i15.1976.
Aktas G, Alcelik A, Tekce BK, Tekelioglu V, Sit M, Savli H. Red cell distribution width and mean platelet volume in patients with irritable bowel syndrome. Prz Gastroenterol. 2014;9:160. https://doi.org/10.5114/pg.2014.43578.
Adler J, Rahal K, Swanson SD, Schmiedlin-Ren P, Rittershaus AC, Reingold LJ, et al. Anti-Tumor Necrosis Factor α Prevents Bowel Fibrosis Assessed by Messenger RNA, Histology, and Magnetization Transfer MRI in Rats With Crohn’s Disease. Inflamm Bowel Dis. 2013;19:683–90. https://doi.org/10.1097/MIB.0b013e3182802c32.
Afsar B, Elsurer R, Yilmaz MI, Eyileten T, Yenicesu M. Irritable bowel syndrome in haemodialysis: Prevalence, link with quality of life and depression. Nephrology. 2010;15:197–202. https://doi.org/10.1111/j.1440-1797.2009.01189.x.
Hatanaka T. Clinical pharmacokinetics of pravastatin: mechanisms of pharmacokinetic events. Clin Pharmacokinet. 2000;39:397–412. https://doi.org/10.2165/00003088-200039060-00002.
Masters BA, Palmoski MJ, Flint OP, Gregg RE, Wangiverson D, Durham SK. In vitro myotoxicity of the 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, pravastatin, lovastatin, and simvastatin, using neonatal rat skeletal myocytes. Toxicol Appl Pharmacol. 1995;131:163–74. https://doi.org/10.1006/taap.1995.1058.
Jiang Z, Yu B, Li Y. Effect of three statins on glucose uptake of cardiomyocytes and its mechanism. Med Sci Monit. 2016;22:2825. https://doi.org/10.12659/MSM.897047.
Chaulin AM. Main analytical characteristics of laboratory methods for the determination of cardiac troponins: A review from the historical and modern points of view. Orv Hetil. 2022;163:12–20. https://doi.org/10.1556/650.2021.32296.
Godoy JC, Niesman IR, Busija AR, Kassan A, Schilling JM, Schwarz A, et al. Atorvastatin, but not pravastatin, inhibits cardiac Akt/mTOR signaling and disturbs mitochondrial ultrastructure in cardiac myocytes. The FASEB Journal. 2019;33:1209. https://doi.org/10.1096/fj.201800876R.
Mendes P, Robles PG, Mathur S. Statin-induced rhabdomyolysis: a comprehensive review of case reports. Physiother Can. 2014;66:124–32. https://doi.org/10.3138/ptc.2012-65.
Sager JE, Yu J, Ragueneau-Majlessi I, Isoherranen N. Physiologically based pharmacokinetic (PBPK) modeling and simulation approaches: a systematic review of published models, applications, and model verification. Drug Metab Dispos. 2015;43:1823–37. https://doi.org/10.1124/dmd.115.065920.
Alsmadi MM, Al Eitan LN, Idkaidek NM, Alzoubi KH. The development of a PBPK model for atomoxetine using levels in plasma, saliva and brain extracellular fluid in patients with normal and deteriorated kidney function. CNS Neurol Disord Drug Targets. 2022;21:704–16. https://doi.org/10.2174/1871527320666210621102437.
Alsmadi MM. Evaluating the Pharmacokinetics of Fentanyl in the Brain Extracellular Fluid, Saliva, Urine, and Plasma of Newborns from Transplacental Exposure from Parturient Mothers Dosed with Epidural Fentanyl Utilizing PBPK Modeling. Eur J Drug Metab Pharmacokinet. 2023;48:567–86. https://doi.org/10.1007/s13318-023-00842-8.
Alsmadi MM, Idkaidek N. The Analysis of Pethidine Pharmacokinetics in Newborn Saliva, Plasma, and Brain Extracellular Fluid After Prenatal Intrauterine Exposure from Pregnant Mothers Receiving Intramuscular Dose Using PBPK Modeling. Eur J Drug Metab Pharmacokinet. 2023;48:281–300. https://doi.org/10.1007/s13318-023-00823-x.
Alsmadi MM. Salivary therapeutic monitoring of methadone toxicity in neonates after transplacental transfer from parturient mothers treated with oral methadone guided by PBPK modeling. Comput Toxicol. 2023;29:100296. https://doi.org/10.1016/j.comtox.2023.100296.
Alsmadi MM. Salivary therapeutic monitoring of buprenorphine in neonates after maternal sublingual dosing guided by physiologically based pharmacokinetic modeling. Ther Drug Monit. 2024. https://doi.org/10.1097/ftd.0000000000001172.
Nestorov I. Whole-body physiologically based pharmacokinetic models. Expert Opin Drug Metab Toxicol. 2007;3:235–49. https://doi.org/10.1517/17425255.3.2.235.
Tsamandouras N, Rostami-Hodjegan A, Aarons L. Combining the ‘bottom up’and ‘top down’approaches in pharmacokinetic modelling: fitting PBPK models to observed clinical data. Br J Clin Pharmacol. 2015;79:48–55. https://doi.org/10.1111/bcp.12234.
Peters SA. Physiologically-based pharmacokinetic (PBPK) modeling and simulations: principles, methods, and applications in the pharmaceutical industry. 2nd ed. Newjersy, USA: John Wiley & Sons; 2021.
Committee for Medicinal Products for Human Use. Guideline on the reporting of physiologically based pharmacokinetic (PBPK) modelling and simulation. 2016. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-reporting-physiologically-based-pharmacokinetic-pbpk-modelling-simulation_en.pdf. Accessed 20 Sept 2023.
Center for Drug Evaluation and Research. Physiologically Based Pharmacokinetic Analyses — Format and Content: Guidance for Industry. 2018 https://www.fda.gov/media/101469/download. Accessed 13 Sept 2023.
Jones H, Chen Y, Gibson C, Heimbach T, Parrott N, Peters S, et al. Physiologically based pharmacokinetic modeling in drug discovery and development: a pharmaceutical industry perspective. Clin Pharmacol Ther. 2015;97:247–62. https://doi.org/10.1002/cpt.37.
Johnson TN, Boussery K, Rowland-Yeo K, Tucker GT, Rostami-Hodjegan A. A semi-mechanistic model to predict the effects of liver cirrhosis on drug clearance. Clin Pharmacokinet. 2010;49:189–206. https://doi.org/10.2165/11318160-000000000-00000.
Ou Y, Xu Y, Gore L, Harvey RD, Mita A, Papadopoulos KP, et al. Physiologically-based pharmacokinetic modelling to predict oprozomib CYP3A drug–drug interaction potential in patients with advanced malignancies. Br J Clin Pharmacol. 2019;85:530–9. https://doi.org/10.1111/bcp.13817.
Cheeti S, Budha NR, Rajan S, Dresser MJ, Jin JY. A physiologically based pharmacokinetic (PBPK) approach to evaluate pharmacokinetics in patients with cancer. Biopharm Drug Disposition. 2013;34:141–54. https://doi.org/10.1002/bdd.1830.
Alsmadi MM, AL‐Daoud NM, Jaradat MM, Alzughoul SB, Abu KAD, Abu LSS, et al. Physiologically‐based pharmacokinetic model for alectinib, ruxolitinib, and panobinostat in the presence of cancer, renal impairment, and hepatic impairment. Biopharm Drug Disposition. 2021; 42, 263-84. https://doi.org/10.1002/bdd.2282.
Watanabe T, Kusuhara H, Maeda K, Shitara Y, Sugiyama Y. Physiologically based pharmacokinetic modeling to predict transporter-mediated clearance and distribution of pravastatin in humans. J Pharmacol Exp Ther. 2009;328:652–62. https://doi.org/10.1124/jpet.108.146647.
Varma MV, Lai Y, Feng B, Litchfield J, Goosen TC, Bergman A. Physiologically based modeling of pravastatin transporter-mediated hepatobiliary disposition and drug-drug interactions. Pharm Res. 2012;29:2860–73. https://doi.org/10.1007/s11095-012-0792-7.
Alsmadi MM, Al-Daoud NM, Obaidat RM, Abu-Farsakh NA. Enhancing atorvastatin in vivo oral bioavailability in the presence of inflammatory bowel disease and irritable bowel syndrome using supercritical fluid technology guided by wbPBPK modeling in rat and human. AAPS PharmSciTech. 2022;23:148. https://doi.org/10.1208/s12249-022-02302-z.
Effinger A, O’Driscoll CM, McAllister M, Fotaki N. Predicting budesonide performance in healthy subjects and patients with Crohn’s disease using biorelevant in vitro dissolution testing and PBPK modeling. Eur J Pharm Sci. 2021;157:105617. https://doi.org/10.1016/j.ejps.2020.105617.
Effinger A, O’Driscoll CM, McAllister M, Fotaki N. Gastrointestinal diseases and their impact on drug solubility: Crohn’s disease. Eur J Pharm Sci. 2020;152:105459. https://doi.org/10.1016/j.ejps.2020.105459.
Beckman Coulter. Albumin kit- Instructions for Use. 2015. https://www.beckmancoulter.com/wsrportal/techdocs?docname=/cis/BAOSR6x02A/01/EN_ALB. Accessed 4 Dec 2023.
Kivistö KT, Grisk O, Hofmann U, Meissner K, Möritz K-U, Ritter C, et al. Disposition of oral and intravenous pravastatin in MRP2-deficient TR–rats. Drug Metab Disposition. 2005;33:1593–6. https://doi.org/10.1124/dmd.105.006262.
Dupont WD, Plummer WD Jr. Power and sample size calculations: a review and computer program. Control Clin Trials. 1990;11:116–28. https://doi.org/10.1016/0197-2456(90)90005-m.
Yamada T, Grisham MB, Deitch E, Specian RD, Perry MA, Sartor RB. Mechanisms of acute and chronic intestinal inflammation induced by indomethacin. Inflammation. 1993;17:641–62. https://doi.org/10.1007/BF00920471.
La J-H, Kim T-W, Sung T-S, Kang J-W, Kim H-J, Yang I-S. Visceral hypersensitivity and altered colonic motility after subsidence of inflammation in a rat model of colitis. World J Gastroenterol WJG. 2003;9:2791. https://doi.org/10.3748/wjg.v9.i12.2791.
Williams CL, Villar RG, Peterson JM, Burks TF. Stress-induced changes in intestinal transit in the rat: a model for irritable bowel syndrome. Gastroenterology. 1988;94:611–21. https://doi.org/10.1016/0016-5085(88)90231-4.
Zhuang Z, Zhang L, Wang X, Tao L, Lv B. PDIA3 gene induces visceral hypersensitivity in rats with irritable bowel syndrome through the dendritic cell-mediated activation of T cells. PeerJ. 2016;4:1–12. https://doi.org/10.7717/peerj.2644.
Diehl KH, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D, et al. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol. 2001;21:15–23. https://doi.org/10.1002/jat.727.
McGuill MW, Rowan AN. Biological effects of blood loss: implications for sampling volumes and techniques. ILAR J. 1989;31:5–20. https://doi.org/10.1093/ilar.31.4.5.
Certara. The industry standard for pharmacokinetic / pharmacodynamic (PK/PD) analysis Phoenix WinNonlin Software,. https://www.certara.com/software/phoenix-winnonlin/. Accessed 22 Nov 2023.
Microsoft Office. Use the Analysis ToolPak to perform complex data analysis. https://support.microsoft.com/en-au/office/use-the-analysis-toolpak-to-perform-complex-data-analysis-6c67ccf0-f4a9-487c-8dec-bdb5a2cefab6. Accessed 22 Nov 2023.
Siegel S. Nonparametric statistics. Am Stat. 1957;11:13–9. https://doi.org/10.1080/00031305.1957.10501091.
Bayer Technology Services GmbH. Open Systems Pharmacology Suite. 2019. http://www.systems-biology.com/products/pk-sim/. Accessed 10 June 2021.
Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci. 2006;95:1238–57. https://doi.org/10.1002/jps.20502.
Kuepfer L, Niederalt C, Wendl T, Schlender JF, Willmann S, Lippert J, et al. Applied concepts in PBPK modeling: how to build a PBPK/PD model. CPT Pharmacometrics Syst Pharmacol. 2016;5:516–31. https://doi.org/10.1002/psp4.12134.
Komai T, Kawai K, Tokui T, Tokui Y, Kuroiwa C, Shigehara E, et al. Disposition and metabolism of pravastatin sodium in rats, dogs and monkeys. Eur J Drug Metab Pharmacokinet. 1992;17:103–13. https://doi.org/10.1007/BF03188778.
Germershausen JI, Hunt VM, Bostedor RG, Bailey PJ, Karkas JD, Alberts AW. Tissue selectivity of the cholesterol-lowering agents lovastatin, simvastatin and pravastatin in rats in vivo. Biochem Biophys Res Commun. 1989;158:667–75. https://doi.org/10.1016/0006-291X(89)92773-3.
Banerjee AK, Peters TJ. Experimental non-steroidal anti-inflammatory drug-induced enteropathy in the rat: similarities to inflammatory bowel disease and effect of thromboxane synthetase inhibitors. Gut. 1990;31:1358–64. https://doi.org/10.1136/gut.31.12.1358.
Corak A, Coşkun T, Alican I, Kurtel H, Yeğen BC. The effect of nitric oxide synthase blockade and indomethacin on gastric emptying and gastric contractility. Pharmacology. 1997;54:298–304. https://doi.org/10.1159/000139499.
Martínez V, Wang L, Taché Y. Proximal colon distension induces Fos expression in the brain and inhibits gastric emptying through capsaicin-sensitive pathways in conscious rats. Brain Res. 2006;1086:168–80. https://doi.org/10.1016/j.brainres.2006.02.063.
Fujiyama N, Shitara Y, Horie T. The mechanism of the down-regulation of hepatic transporters in rats with indomethacin-induced intestinal injury. Dig Dis Sci. 2013;58:1891–8. https://doi.org/10.1007/s10620-013-2587-z.
Rowland Yeo K, Aarabi M, Jamei M, Rostami-Hodjegan A. Modeling and predicting drug pharmacokinetics in patients with renal impairment. Expert Rev Clin Pharmacol. 2011;4:261–74. https://doi.org/10.1586/ecp.10.143.
Berezhkovskiy LM. Volume of distribution at steady state for a linear pharmacokinetic system with peripheral elimination. J Pharm Sci. 2004;93:1628–40. https://doi.org/10.1002/jps.20073.
Rodgers T, Jones HM, Rowland M. Tissue lipids and drug distribution: dog versus rat. J Pharm Sci. 2012;101:4615–26. https://doi.org/10.1002/jps.23285.
Kyrklund C, Backman JT, Neuvonen M, Neuvonen PJ. Effect of rifampicin on pravastatin pharmacokinetics in healthy subjects. Br J Clin Pharmacol. 2004;57:181–7. https://doi.org/10.1046/j.1365-2125.2003.01972.x.
Jacobsen W, Kuhn B, Soldner A, Kirchner G, Sewing K-F, Kollman PA, et al. Lactonization is the critical first step in the disposition of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor atorvastatin. Drug Metab Dispos. 2000;28:1369–78.
Igel M, Arnold KA, Niemi M, Hofmann U, Schwab M, Lütjohann D, et al. Impact of the SLCO1B1 polymorphism on the pharmacokinetics and lipid-lowering efficacy of multiple-dose pravastatin. Clin Pharmacol Ther. 2006;79:419–26. https://doi.org/10.1016/j.clpt.2006.01.010.
Maharaj AR, Barrett J, Edginton AN. A workflow example of PBPK modeling to support pediatric research and development: case study with lorazepam. AAPS J. 2013;15:455–64. https://doi.org/10.1208/s12248-013-9451-0.
Sampson MR, Frymoyer A, Rattray B, Cotten CM, Smith PB, Capparelli E, et al. Predictive performance of a gentamicin population pharmacokinetic model in neonates receiving full-body hypothermia. Ther Drug Monit. 2014;36:584. https://doi.org/10.1097/FTD.0000000000000056.
FDA. Bioanalytical Method Validation Guidance for Industry. 2009. https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf. Accessed 15 Nov 2023.
FDA bioequivalence standards. AAPS Advances in the Pharmaceutical Sciences Series 13. MD, USA: Springer; 2014.
Chen L, Joshi P, Piatkivskyi A, Aguilar K, Lin J. Method development and validation for the determination of pravastatin in human plasma by LC-MS/MS. J Bioanal Biomed. 2017; 9. https://doi.org/10.4172/1948-593x.1000168
Martinez MN, Amidon GL. A mechanistic approach to understanding the factors affecting drug absorption: a review of fundamentals. J Clin Pharmacol. 2002;42:620–43. https://doi.org/10.1177/00970002042006005.
Peters SA. Physiologically based pharmacokinetic (PBPK) modeling and simulations: principles, methods, and applications in the pharmaceutical industry. John Wiley & Sons; 2021.
Cui M, Zhang M, Liu K. Colon-targeted drug delivery of polysaccharide-based nanocarriers for synergistic treatment of inflammatory bowel disease: A review. Carbohydr Polym. 2021;272:118530. https://doi.org/10.1016/j.carbpol.2021.118530.
Lozoya-Agullo I, Gonzalez-Alvarez I, Zur M, Fine-Shamir N, Cohen Y, Markovic M, et al. Closed-Loop Doluisio (Colon, Small Intestine) and Single-Pass Intestinal Perfusion (Colon, Jejunum) in Rat—Biophysical Model and Predictions Based on Caco-2. Pharm Res. 2017;35:2. https://doi.org/10.1007/s11095-017-2331-z.
Awad K, Barmeyer C, Bojarski C, Nagel O, Lee I-FM, Schweiger MR, et al. Impaired intestinal permeability of tricellular tight junctions in patients with irritable bowel syndrome with mixed bowel habits (IBS-M). Cells. 2023; 12: 236. https://doi.org/10.3390/cells12020236.
Söderholm JD, Peterson KH, Olaison G, Franzén LE, Weström B, Magnusson K-E, et al. Epithelial permeability to proteins in the noninflamed ileum of Crohn’s disease? Gastroenterology. 1999;117:65–72. https://doi.org/10.1016/S0016-5085(99)70551-2.
Söderholm JD, Streutker C, Yang PC, Paterson C, Singh PK, McKay DM, et al. Increased epithelial uptake of protein antigens in the ileum of Crohn’s disease mediated by tumour necrosis factor alpha. Gut. 2004;53:1817–24. https://doi.org/10.1136/gut.2004.041426.
Press AG, Hauptmann IA, Hauptmann L, Fuchs B, Fuchs M, Ewe K, et al. Gastrointestinal pH profiles in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 1998;12:673–8. https://doi.org/10.1046/j.1365-2036.1998.00358.x.
De Schepper H, De Man JG, Nassauw LV, Timmermans J-P, Herman A, Pelckmans P, et al. Acute distal colitis impairs gastric emptying in rats via an extrinsic neuronal reflex pathway involving the pelvic nerve. Gut. 2007;56:195–202. https://doi.org/10.1136/gut.2006.104745.
Harris NR, Carter PR, Lee S, Watts MN, Zhang S, Grisham MB. Association between blood flow and inflammatory state in a T-cell transfer model of inflammatory bowel disease in mice. Inflamm Bowel Dis. 2010;16:776–82. https://doi.org/10.1002/ibd.21126.
Zhuang X, Lu C. PBPK modeling and simulation in drug research and development. Acta Pharmaceutica Sinica B. 2016;6:430–40. https://doi.org/10.1016/j.apsb.2016.04.004.
Chow ECY, Talattof A, Tsakalozou E, Fan J, Zhao L, Zhang X. Using physiologically based pharmacokinetic (PBPK) modeling to evaluate the impact of pharmaceutical excipients on oral drug absorption: sensitivity analyses. The AAPS journal. 2016;18:1500–11. https://doi.org/10.1208/s12248-016-9964-4.
Wu LX, Guo CX, Chen WQ, Yu J, Qu Q, Chen Y, et al. Inhibition of the organic anion-transporting polypeptide 1B1 by quercetin: an in vitro and in vivo assessment. Br J Clin Pharmacol. 2012;73:750–7. https://doi.org/10.1111/j.1365-2125.2011.04150.x.
Kyrklund C, Backman JT, Neuvonen M, Neuvonen PJ. Gemfibrozil increases plasma pravastatin concentrations and reduces pravastatin renal clearance. Clin Pharmacol Ther. 2003;73:538–44. https://doi.org/10.1016/S0009-9236(03)00052-3.
Nakagomi-Hagihara R, Nakai D, Tokui T. Inhibition of human organic anion transporter 3 mediated pravastatin transport by gemfibrozil and the metabolites in humans. Xenobiotica. 2007;37:416–26. https://doi.org/10.1080/00498250601188808.
Wang L, Prasad B, Salphati L, Chu X, Gupta A, Hop CE, et al. Interspecies variability in expression of hepatobiliary transporters across human, dog, monkey, and rat as determined by quantitative proteomics. Drug Metab Disposition. 2015;43:367–74. https://doi.org/10.1124/dmd.114.061580.
Cheung KWK, van Groen BD, Spaans E, van Borselen MD, de Bruijn AC, Simons-Oosterhuis Y, et al. A comprehensive analysis of ontogeny of renal drug transporters: mRNA analyses, quantitative proteomics, and localization. Clin Pharmacol Ther. 2019;106:1083–92. https://doi.org/10.1002/cpt.1516.
Soetaert K, Petzoldt T. Inverse modelling, sensitivity and monte carlo analysis in R using package FME. J Stat are. 2010; 33: 1-28. https://doi.org/10.18637/jss.v033.i03.
Rowland M, Tozer TN. Clinical Pharmacokinetics and Pharmacodynamics: Concepts and Applications. 5th ed. Philadelphia, USA: Lippincott Williams and Wilkins; 2005.
Wang YT, Mohammed SD, Farmer AD, Wang D, Zarate N, Hobson AR, et al. Regional gastrointestinal transit and pH studied in 215 healthy volunteers using the wireless motility capsule: influence of age, gender, study country and testing protocol. Aliment Pharmacol Ther. 2015;42:761–72. https://doi.org/10.1111/apt.13329.
Tatsuta M, Iishi H, Okuda S. Gastric emptying in patients with fundal gastritis and gastric cancer. Gut. 1990;31:767–9. https://doi.org/10.1136/gut.31.7.767.
Benini F, Mora A, Turini D, Bertolazzi S, Lanzarotto F, Ricci C, et al. Slow gallbladder emptying reverts to normal but small intestinal transit of a physiological meal remains slow in celiac patients during gluten-free diet. Neurogastroenterol Motil. 2012;24:100-e80. https://doi.org/10.1111/j.1365-2982.2011.01822.x.
Miyaji, Azuma, Ito, Abe, Ono, Suto, et al. The effect of Helicobacter pylori eradication therapy on gastric antral myoelectrical activity and gastric emptying in patients with non‐ulcer dyspepsia. Aliment Pharmacol Ther. 1999; 13: 1303-9. https://doi.org/10.1046/j.1365-2036.1999.00621.x.
Marathe CS, Rayner CK, Jones KL, Horowitz M. Relationships between gastric emptying, postprandial glycemia, and incretin hormones. Diabetes Care. 2013;36:1396–405. https://doi.org/10.2337/dc12-1609.
Levi S, Beardshall K, Swift I, Foulkes W, Playford R, Ghosh P, et al. Antral Helicobacter pylori, hypergastrinaemia, and duodenal ulcers: effect of eradicating the organism. BMJ. 1989;299:1504–5. https://doi.org/10.1136/bmj.299.6714.1504.
Ellis LC, Hawksworth GM, Weaver RJ. ATP-dependent transport of statins by human and rat MRP2/Mrp2. Toxicol Appl Pharmacol. 2013;269:187–94. https://doi.org/10.1016/j.taap.2013.03.019.
Wagner JB, Ruggiero M, Leeder JS, Hagenbuch B. Functional consequences of pravastatin isomerization on OATP1B1-mediated transport. Drug Metab Disposition. 2020;48:1192–8. https://doi.org/10.1016/j.taap.2013.03.019.
Watanabe T, Kusuhara H, Watanabe T, Debori Y, Maeda K, Kondo T, et al. Prediction of the overall renal tubular secretion and hepatic clearance of anionic drugs and a renal drug-drug interaction involving organic anion transporter 3 in humans by in vitro uptake experiments. Drug Metab Disposition. 2011;39:1031–8. https://doi.org/10.1124/dmd.110.036129.
Mathialagan S, Piotrowski MA, Tess DA, Feng B, Litchfield J, Varma MV. Quantitative prediction of human renal clearance and drug-drug interactions of organic anion transporter substrates using in vitro transport data: a relative activity factor approach. Drug Metab Disposition. 2017;45:409–17. https://doi.org/10.1124/dmd.116.074294.
Doerksen MJ, Jones RS, Coughtrie MW, Collier AC. Parameterization of microsomal and cytosolic scaling factors: Methodological and biological considerations for scalar derivation and validation. Eur J Drug Metab Pharmacokinet. 2021;46:173–83. https://doi.org/10.1007/s13318-020-00666-w.
Jacobson TA. Comparative pharmacokinetic interaction profiles of pravastatin, simvastatin, and atorvastatin when coadministered with cytochrome P450 inhibitors. Am J Cardiol. 2004;94:1140–6. https://doi.org/10.1016/j.amjcard.2004.07.080.
Hatanaka T, Honda S, Sasaki S, Katayama K, Koizumi T. Pharmacokinetic and pharmacodynamic evaluation for tissue-selective inhibition of cholesterol synthesis by pravastatin. J Pharmacokinet Biopharm. 1998;26:329–47. https://doi.org/10.1023/A:1023237510458.
Acknowledgements
The authors acknowledge Jordan University of Science and Technology (Irbid, Jordan) and the University of Petra (Amman, Jordan) for all the facilities and support provided. Also, the authors acknowledge Triumpharma (Amman, Jordan) for analyzing pravastatin levels in rats in vivo studies.
Funding
This project was funded by the Jordan University of Science and Technology [local fund number: 53/2022] and the University of Petra [local fund number: 2/4/2023]
Author information
Authors and Affiliations
Contributions
Alsmadi, Abudaqqa, and Idkaidek had substantial contributions to the conception and design of the work; analysis and interpretation of data, drafting of the work, and revising it critically for important intellectual content. All authors had substantial contributions to the acquisition of data, final approval of the version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy and integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing Interests
None declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alsmadi, M.M., Abudaqqa, A.A., Idkaidek, N. et al. The Effect of Inflammatory Bowel Disease and Irritable Bowel Syndrome on Pravastatin Oral Bioavailability: In vivo and in silico evaluation using bottom-up wbPBPK modeling. AAPS PharmSciTech 25, 86 (2024). https://doi.org/10.1208/s12249-024-02803-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-024-02803-z