Abstract
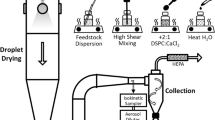
Pressurized metered dose inhalers (pMDIs) require optimized formulations to provide stable, consistent lung delivery. This study investigates the feasibility of novel rugose lipid particles (RLPs) as potential drug carriers in pMDI formulations. The physical stability of RLPs was assessed in three different propellants: the established HFA-134a and HFA-227ea and the new low global-warming-potential (GWP) propellant HFO-1234ze. A feedstock containing DSPC and calcium chloride was prepared without pore forming agent to spray dry two RLP batches at inlet temperatures of 55 °C (RLP55) and 75 °C (RLP75). RLPs performance in pMDI formulations was compared to two reference samples that exhibit significantly different performance when suspended in propellants: well-established engineered porous particles and particles containing 80% trehalose and 20% leucine (80T20L). An accelerated stability study at 40 °C and relative humidity of 7% ± 5% was conducted over 3 months. At different time points, a shadowgraphic imaging technique was used to evaluate the colloidal stability of particles in pMDIs. Field emission electron microscopy with energy dispersive X-ray spectroscopy was used to evaluate the morphology and elemental composition of particles extracted from the pMDIs. After 2 weeks, all 80T20L formulations rapidly aggregated upon agitation and exhibited significantly inferior colloidal stability compared to the other samples. In comparison, both the RLP55 and RLP75 formulations, regardless of the propellant used, retained their rugose structure and demonstrated excellent suspension stability comparable with the engineered porous particles. The studied RLPs demonstrate great potential for use in pMDI formulations with HFA propellants and the next-generation low-GWP propellant HFO-1234ze.
Graphical Abstract











Similar content being viewed by others
References
Xie M, Liu X, Cao X, Guo M, Li X. Trends in prevalence and incidence of chronic respiratory diseases from 1990 to 2017. Respir Res. 2020;21. https://doi.org/10.1186/s12931-020-1291-8.
Newman SP. Delivering drugs to the lungs: The history of repurposing in the treatment of respiratory diseases. Adv Drug Deliv Rev. 2018;133:5–18. https://doi.org/10.1016/j.addr.2018.04.010.
Vehring R. Pharmaceutical particle engineering via spray drying. Pharm Res. 2008;25:999–1022. https://doi.org/10.1007/s11095-007-9475-1.
Martin AR. Regional deposition: targeting. J Aerosol Med Pulm Drug Deliv. 2021;34:1–10. https://doi.org/10.1089/jamp.2021.29033.am.
Ari A, Fink JB. Recent advances in aerosol devices for the delivery of inhaled medications. Expert Opin Drug Deliv. 2020;17:133–44. https://doi.org/10.1080/17425247.2020.1712356.
Stein SW, Thiel CG. The history of therapeutic aerosols: a chronological review. J Aerosol Med Pulm Drug Deliv. 2017;30:20–41. https://doi.org/10.1089/jamp.2016.1297.
Jones SA. Suspension versus solution metered dose inhalers: different products, different particles? J Drug Deliv Sci Technol. 2011;21:319–22. https://doi.org/10.1016/S1773-2247(11)50049-8.
Environmental Investigation Agency. High stakes: implementing and strengthening climate and ozone commitments under the Montreal Protocol. 2019. https://us.eia.org/report/high-stakes-implementing-and-strengthening-climate-and-ozone-commitments-under-the-montreal-protocol/. Accessed 18 May 2023.
Panigone S, Sandri F, Ferri R, Volpato A, Nudo E, Nicolini G. Environmental impact of inhalers for respiratory diseases: decreasing the carbon footprint while preserving patient-tailored treatment. BMJ Open Respir Res. 2020;7. https://doi.org/10.1136/bmjresp-2020-000571.
Yang L, da Rocha SRP. Understanding solvation in the low global warming hydrofluoroolefin HFO-1234ze propellant. J Phys Chem. 2014;118:10675–87. https://doi.org/10.1021/jp5059319.
Wilkinson A, Woodcock A. The environmental impact of inhalers for asthma: a green challenge and a golden opportunity. Br J Clin Pharmacol. 2021;88:3015–22. https://doi.org/10.1111/bcp.15135.
Pritchard JN. The climate is changing for metered-dose inhalers and action is needed. Drug Des Devel Ther. 2020;14:3043–55. https://doi.org/10.2147/DDDT.S262141.
Myrdal PB, Sheth P, Stein SW. Advances in metered dose inhaler technology: formulation development. AAPS PharmSciTech. 2014;15:434–55. https://doi.org/10.1208/s12249-013-0063-x.
Traini D, Young PM, Rogueda P, Price R. In vitro investigation of drug particulates interactions and aerosol performance of pressurized metered dose inhalers. Pharm Res. 2007;24:125–35. https://doi.org/10.1007/s11095-006-9130-2.
Chunhachaichana C, Sawatdee S, Rugmai S, Srichana T. Development and characterization of nanodispersion-based sildenafil pressurized metered-dose inhaler using combined small-angle X-ray scattering, dynamic light scattering, and impactors. J Drug Deliv Sci Technol. 2022;76. https://doi.org/10.1016/j.jddst.2022.103749.
Heyder J. Deposition of inhaled particles in the human respiratory tract and consequences for regional targeting in respiratory drug delivery. ATS. 2004;1:315–20. https://doi.org/10.1513/pats.200409-046TA.
Cocks E, Somavarapu S, Alpar O, Greenleaf D. Influence of suspension stabilisers on the delivery of protein-loaded porous poly (DL-lactide-co-glycolide) (PLGA) microparticles via pressurised metered dose inhaler (pMDI). Pharm Res. 2014;31:2000–9. https://doi.org/10.1007/s11095-014-1302-x.
Saleem IY, Smyth HDC. Tuning aerosol particle size distribution of metered dose inhalers using cosolvents and surfactants. Biomed Res Int. 2013;2013. https://doi.org/10.1155/2013/574310.
Wang H, Tan P, Barona D, Li G, Hoe S, Lechuga-Ballesteros D, et al. Characterization of the suspension stability of pharmaceuticals using a shadowgraphic imaging method. Int J Pharm. 2018;548:128–38. https://doi.org/10.1016/j.ijpharm.2018.06.053.
Williams RL, Adams WP, Poochikian G, Hauck WW. Content uniformity and dose uniformity: current approaches, statistical analyses, and presentation of an alternative approach, with special reference to oral inhalation and nasal drug products. Pharm Res. 2002;19:359–66. https://doi.org/10.1023/A:1015114821387.
Corzo C, Fuchsbichler A, Savencu I, Afonso Urich J, Zimmer A, Lochmann D, et al. Lipid-microparticles for pulmonary delivery of active pharmaceutical ingredients: Impact of lipid crystallization on spray-drying processability. Int J Pharm. 2021;610. https://doi.org/10.1016/j.ijpharm.2021.121259.
Wauthoz N, Amighi K. Phospholipids in pulmonary drug delivery. Eur J Lipid Sci Technol. 2014;116:1114–28. https://doi.org/10.1002/ejlt.201300368.
Duddu SP, Sisk SA, Walter YH, Tarara TE, Trimble KR, Clark AR, et al. Improved lung delivery from a passive dry powder inhaler using an engineered PulmoSphere powder. Pharm Res. 2002;19:689–95. https://doi.org/10.1023/A:1015322616613.
Vehring R, Lechuga-Ballesteros D, Joshi V, Noga B, Dwivedi SK. Cosuspensions of microcrystals and engineered microparticles for uniform and efficient delivery of respiratory therapeutics from pressurized metered dose inhalers. Langmuir. 2012;28:15015–23. https://doi.org/10.1021/la302281n.
Weers JG, Miller DP, Tarara TE. Spray-dried PulmoSphere™ formulations for inhalation comprising crystalline drug particles. AAPS PharmSciTech. 2019;20(3):103. https://doi.org/10.1208/s12249-018-1280-0.
Weers J, Tarara T. The PulmoSphereTM platform for pulmonary drug delivery. Ther Deliv. 2014;5:277–95. https://doi.org/10.4155/tde.14.3.
Wang H, Ordoubadi M, Connaughton P, Lachacz K, Carrigy N, Tavernini S, et al. Spray dried rugose lipid particle platform for respiratory drug delivery. Pharm Res. 2022;39:805–23. https://doi.org/10.1007/s11095-022-03242-w.
Wang H, Connaughton P, Lachacz K, Carrigy N, Ordoubadi M, Lechuga-Ballesteros D, et al. Inhalable microparticle platform based on a novel shell-forming lipid excipient and its feasibility for respirable delivery of biologics. Eur J Pharm Biopharm. 2022;177:308–22. https://doi.org/10.1016/j.ejpb.2022.07.013.
Hoe S, Ivey JW, Boraey MA, Shamsaddini-Shahrbabak A, Javaheri E, Matinkhoo S, Finlay WH, Vehring R. Use of a fundamental approach to spray-drying formulation design to facilitate the development of multi-component dry powder aerosols for respiratory drug delivery. Pharm Res. 2014;31:449–65. https://doi.org/10.1007/s11095-013-1174-5.
Ivey JW. Particle formation from evaporating microdroplets for inhaled drug delivery. PhD Thesis, Mech. Eng, University of Alberta, Edmonton, Alberta. 2018. https://doi.org/10.7939/R3RB6WJ3S.
Decaire B, Conviser S. Materials compatibility testing of Honeywell’s new low global warming potential propellants. Aptar Pharma, France. 2011. https://sustainability.honeywell.com/content/dam/sustainability/en/documents/document-lists/technical/poster-honeywell-propellants.pdf. Accessed 18 Jul 2023.
Daikin Industries Ltd. SolkaneTM 227 and 134a pharma. https://www.daikinchem.de/products-and-performance/pharma-propellants. Accessed 18 Jul 2023.
Honeywell. HFO-1234ze(E) Safety data sheet. 2021. https://www.honeywellsds.com. Accessed 18 Jul 2023
Smith C, Nicholls ZRJ, Armour K, Collins W, Forster P, Meinshausen M, Palmer MD, Watanabe M. The earth’s energy budget, climate feedbacks, and climate sensitivity supplementary material. In Climate Change 2021: The Physical Science Basis. 2021. https://www.ipcc.ch/.
Ivey JW, Bhambri P, Church TK, Lewis DA, McDermott MT, Elbayomy S, et al. Humidity affects the morphology of particles emitted from beclomethasone dipropionate pressurized metered dose inhalers. Int J Pharm. 2017;520:207–15. https://doi.org/10.1016/j.ijpharm.2017.01.062. (Elsevier B.V.).
Wang H, Tan P, Barona D, Li G, et al. A new shadowgraphic imaging method for the suspension stability analysis of pressurized metered dose inhalers. RDD. Tucson, Arizona, US. 2018;2:573–78. https://www.rddonline.com/rdd/article.php?id=0&sid=103&ArticleID=2420&return=1. Accessed 5 Mar 2024.
Wang H, Bhambri P, Ivey J, Vehring R. Design and pharmaceutical applications of a low-flow-rate single-nozzle impactor. Int J Pharm. 2017;533:14–25. https://doi.org/10.1016/j.ijpharm.2017.09.047.
Wang H, Nobes DS, Vehring R. Particle surface roughness improves colloidal stability of pressurized pharmaceutical suspensions. Pharm Res. 2019;36. https://doi.org/10.1007/s11095-019-2572-0.
Flament MP, Leterme P, Gayot A. The influence of carrier roughness on adhesion, content uniformity and the in vitro deposition of terbutaline sulphate from dry powder inhalers. Int J Pharm. 2004;275:201–9. https://doi.org/10.1016/j.ijpharm.2004.02.002.
Lechuga-Ballesteros D, Noga B, Vehring R, Cummings RH, Dwivedi SK. Novel cosuspension metered-dose inhalers for the combination therapy of chronic obstructive pulmonary disease and asthma. Future Med Chem. 2011;3:1703–18. https://doi.org/10.4155/fmc.11.133.
Carrigy NB, Ordoubadi M, Liu Y, Melhem O, Barona D, Wang H, et al. Amorphous pullulan trehalose microparticle platform for respiratory delivery. Int J Pharm. 2019;563:156–68. https://doi.org/10.1016/j.ijpharm.2019.04.004.
Carrigy N, Vehring R. Engineering stable spray-dried biologic powder for inhalation. In: Pharmaceutical Inhalation Aerosol Technology. CRC Press. 2019. https://doi.org/10.1201/9780429055201-12.
Gomez M, McCollum J, Wang H, Ordoubadi M, Jar C, Carrigy NB, et al. Development of a formulation platform for a spray-dried, inhalable tuberculosis vaccine candidate. Int J Pharm. 2021;593. https://doi.org/10.1016/j.ijpharm.2020.120121.
Ordoubadi M, Shepard KB, Wang H, Wang Z, Pluntze AM, Churchman JP, et al. On the physical stability of leucine-containing spray-dried powders for respiratory drug delivery. Pharmaceutics. 2023;15:435. https://doi.org/10.3390/pharmaceutics15020435.
Ordoubadi M, Gregson FKA, Wang H, Nicholas M, Gracin S, Lechuga-Ballesteros D, et al. On the particle formation of leucine in spray drying of inhalable microparticles. Int J Pharm. 2021;592. https://doi.org/10.1016/j.ijpharm.2020.120102.
FDA. Metered dose inhaler (MDI) and dry powder inhaler (DPI) products-quality considerations guidance for industry; draft guidance. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/metered-dose-inhaler-mdi-and-dry-powder-inhaler-dpi-drug-products-quality-considerations. Accessed 5 Mar 2024.
Wang H, Nobes DS, Finlay WH, Vehring R. Agitation method affects colloidal stability of pharmaceutical suspensions. RDD. Lisbon, Portugal. 2019;2:425–30. https://www.rddonline.com/rdd/article.php?ArticleID=2634. Accessed 5 Mar 2024.
Usmani OS, Roche N, Jenkins M, Stjepanovic N, Mack P, De Backer W. Consistent pulmonary drug delivery with whole lung deposition using the aerosphere inhaler: a review of the evidence. Int J Chron Obstruct Pulmon Dis. 2021;16:113–24. https://doi.org/10.2147/COPD.S274846.
Lechuga-Ballesteros D, Vehring R, Dwivedi SK. A new co-suspension mdi platform: scientific foundations of mono, dual and triple combination products. RDD. Berlin, Germany. 2011;1:101–12. https://www.rddonline.com/rdd/article.php?id=0&sid=103&ArticleID=1580&return=1. Accessed 5 Mar 2024.
Weers JG, Miller DP, Tarara TE. Spray-dried PulmoSphereTM formulations for inhalation comprising crystalline drug particles. AAPS PharmSciTech. 2019;20:103. https://doi.org/10.1208/s12249-018-1280-0.
D’Sa D, Chan HK, Kim HW, Chrzanowski W. Quantitative and qualitative examination of particle-particle interactions using colloidal probe nanoscopy. J Vis Exp. 2014;89:51874. https://doi.org/10.3791/51874.
Wolska E. Fine powder of lipid microparticles – spray drying process development and optimization. J Drug Deliv Sci Technol. 2021;64. https://doi.org/10.1016/j.jddst.2021.102640.
Wang H, Ordoubadi M, Leal J, Minootan A, Lachacz K, Carrigy NB, Lechuga-Ballesteros D, Vehring R. RDD. Antibes, France. 2023;2023:383–86. https://www.rddonline.com/rdd/article.php?ArticleID=2982&id=22. Accessed 5 Mar 2024.
Weers JG, Tarara TE, Dellamary LA et al. US7442388. 2008.
Williams RO, Hu C. Moisture uptake and its influence on pressurized metered-dose inhalers. Pharm Dev Technol. 2000;5:153–62. https://doi.org/10.1081/PDT-100100530.
Rossi I, Ganley WJ, Chi P, Kwok L, Zadrazil I, Hassall G, et al. Fundamental properties of propellant aerosols can guide transition to low global warming potential pMDIs: size, velocity and surface charge. DDL. 2021;32. https://ddl-conference.com/ddl2021/conference-papers/fundamental-properties-of-propellant-aerosols-can-guide-transition-to-low-global-warming-potential-pmdis-size-velocity-and-surface-charge/. Accessed 5 Mar 2024.
Amaro MI, Tajber L, Corrigan OI, Healy AM. Co-Spray dried carbohydrate microparticles: Crystallisation delay/inhibition and improved aerosolization characteristics through the incorporation of hydroxypropyl-β-cyclodextrin with amorphous raffinose or trehalose. Pharm Res. 2015;32:180–95. https://doi.org/10.1007/s11095-014-1454-8.
Murata S, Izumi T, Ito H. Effect of the moisture content in aerosol on the spray performance of Stmerin ® D hydrofluoroalkane preparations (2). Chem Pharm Bull (Tokyo). 2012;60(5):593–7. https://doi.org/10.1248/cpb.60.593.
Acknowledgements
The authors acknowledge the language editing provided by Luba Slabyj.
Funding
This work was supported by AstraZeneca Pharmaceuticals and the Natural Sciences and Engineering Research Council of Canada through a Collaborative Research & Development Program (Grant CRDJP 543336–19).
Author information
Authors and Affiliations
Contributions
Z.M.: conceptualization, writing-original draft preparation, literature search, data acquisition, and analysis. H.W.: conceptualization, analysis, review, editing, and guidance. P.C.: review and editing. K.L: review and editing. N.C.: review and editing. M.O.: review and editing. D.L.-B.: review, editing, guidance. A.R.M. and R.V.: conceptualization, review, editing, supervision, resources and funding, and guidance.
Corresponding author
Ethics declarations
Conflict of Interest
PC, KL, NC, and DLB are employees of AstraZeneca and may own stock or stock options.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Minootan, Z., Wang, H., Connaughton, P. et al. On the Feasibility of Rugose Lipid Microparticles in Pressurized Metered Dose Inhalers with Established and New Propellants. AAPS PharmSciTech 25, 82 (2024). https://doi.org/10.1208/s12249-024-02776-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-024-02776-z