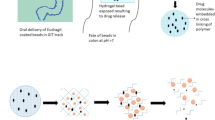
Abstract
Mesalamine is a first-line drug for the treatment of inflammatory bowel diseases. However, its premature release associated with marketed formulations leads to adverse effects like gastric trouble, vomiting, and diarrhoea. To minimize these side effects, colon-targeted drug delivery is essential. Besides conventional pharmacotherapy, bifidogenic probiotics with anti-inflammatory activity has been reported to elicit a significant impact on the remission of ulcerative colitis. Bifidogenic probiotics being acid-labile necessitate developing a gastro-resistant formulation for enhancing the delivery of viable cells to the colon. The present study was aimed at developing a fixed-dose unit dosage form of mucoadhesive hydrogel beads loaded with mesalamine and Bifidobacterium bifidum further encapsulated in Eudragit® capsules for the targeted drug delivery at colonic pH. The hydrogel beads were prepared by ionotropic gelation, with the effect of single and dual-crosslinking approaches on various formulation characteristics studied. Standard size 00 Eudragit® gastro-resistant capsules were prepared and the dried beads were filled inside the capsule shells. The formulation was then evaluated for various parameters, including physicochemical characterization, in vitro biocompatibility and anti-inflammatory activity. No interaction was observed between the drug and the polymers, as confirmed through FTIR, XRD, and DSC analysis. The mean particle size of the beads was ~ 457–485 µm. The optimized formulation showed a drug entrapment efficiency of 95.4 ± 2.58%. The Eudragit® capsule shells disintegrated in approximately 13 min at pH 7.4. The mucoadhesive hydrogel beads sustained the drug release above 18 h, with 50% of the drug released by the end of 12 h. The optimized formulation demonstrated significant (p < 0.05) gastro-resistance, biocompatibility, sustained drug release, cell viability, and anti-inflammatory activity.
Graphical Abstract
















Similar content being viewed by others
Data availability
Additional data will be made available on request.
References
Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1–106. Available from: https://pubmed.ncbi.nlm.nih.gov/31562236/
Horwitz RI, Lobitz G, Mawn M, Conroy AH, Cullen MR, Sim I, et al. Biosocial medicine: Biology, biography, and the tailored care of the patient. SSM - Popul Heal. 2021;15:100863.
Vaghani SS, Patel MM. pH-sensitive hydrogels based on semi-interpenetrating network (semi-IPN) of chitosan and polyvinyl pyrrolidone for clarithromycin release. https://doi.org/10.3109/036390452011563422. 2011;37:1160–9. Available from: https://www.tandfonline.com/doi/abs/https://doi.org/10.3109/03639045.2011.563422
Hughes KA, Misra B, Maghareh M, Bobbala S. Use of stimulatory responsive soft nanoparticles for intracellular drug delivery. Nano Res. 2023;16. Available from: https://pubmed.ncbi.nlm.nih.gov/36685637/
Saiyad M, Shah N. Nanopolymers in drug delivery system. Mater Today Proc. 2022
Sehgal P, Colombel JF, Aboubakr A, Narula N. Systematic review: safety of mesalazine in ulcerative colitis. Aliment Pharmacol Ther. 2018;47:1597–609. Available from: https://pubmed.ncbi.nlm.nih.gov/29722441/
Lim WC, Wang Y, Macdonald JK, Hanauer S. Aminosalicylates for induction of remission or response in Crohn’s disease. Cochrane Database Syst Rev. 2016;2016. Available from: /pmc/articles/PMC6457996/
Yasmin F, Najeeb H, Shaikh S, Hasanain M, Naeem U, Moeed A, et al. Novel drug delivery systems for inflammatory bowel disease. World J Gastroenterol. 2022;28:1922–33. Available from: https://pubmed.ncbi.nlm.nih.gov/35664964/
Ham M, Moss AC. Mesalamine in the treatment and maintenance of remission of ulcerative colitis. Expert Rev Clin Pharmacol. 2012;5:113. Available from: /pmc/articles/PMC3314328/
Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnet PWJ. Psychobiotics and the Manipulation of Bacteria–Gut–Brain Signals. Trends Neurosci. 2016;39:763. Available from: /pmc/articles/PMC5102282/
Venegas DP, De La Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, et al. Short chain fatty acids (SCFAs)mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10:277.
Muller JA, Stanton C, Sybesma W, Fitzgerald GF, Ross RP. Reconstitution conditions for dried probiotic powders represent a critical step in determining cell viability. J Appl Microbiol. 2010;108:1369–79. Available from: https://onlinelibrary.wiley.com/doi/full/https://doi.org/10.1111/j.1365-2672.2009.04533.x
Tankou SK, Regev K, Healy BC, Tjon E, Laghi L, Cox LM, et al. A probiotic modulates the microbiome and immunity in multiple sclerosis. Ann Neurol. 2018;83:1147–61. Available from: https://pubmed.ncbi.nlm.nih.gov/29679417/
Zheng N, Gao Y, Zhu W, Meng D, Allan Walker W. Short chain fatty acids produced by colonizing intestinal commensal bacterial interaction with expressed breast milk are anti-inflammatory in human immature enterocytes. PLoS One. 2020;15:e0229283. Available from: https://journals.plos.org/plosone/article?id=https://doi.org/10.1371/journal.pone.0229283
Kumar H, Collado MC, Wopereis H, Salminen S, Knol J, Roeselers G. The Bifidogenic Effect Revisited—Ecology and Health Perspectives of Bifidobacterial Colonization in Early Life. Microorganisms. 2020;8:1–20. Available from: /pmc/articles/PMC7760687/
Ruiz L, Delgado S, Ruas-Madiedo P, Sánchez B, Margolles A. Bifidobacteria and their molecular communication with the immune system. Front Microbiol. 2017;8:2345.
Andriantsoanirina V, Allano S, Butel MJ, Aires J. Tolerance of Bifidobacterium human isolates to bile, acid and oxygen. Anaerobe. 2013;21:39–42.
Plaza-Díaz J, Ruiz-Ojeda FJ, Vilchez-Padial LM, Gil A. Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases. Nutrients. 2017;9. Available from: https://pubmed.ncbi.nlm.nih.gov/28555037/
Venturi A, Gionchetti P, Rizzello F, Johansson R, Zucconi E, Brigidi P, et al. Impact on the composition of the faecal flora by a new probiotic preparation: preliminary data on maintenance treatment of patients with ulcerative colitis. Aliment Pharmacol Ther. 1999;13:1103–8. Available from: https://pubmed.ncbi.nlm.nih.gov/10468688/
Tursi A, Brandimarte G, Papa A, Giglio A, Elisei W, Giorgetti GM, et al. Treatment of Relapsing Mild-to-Moderate Ulcerative Colitis With the Probiotic VSL#3 as Adjunctive to a Standard Pharmaceutical Treatment: A Double-Blind, Randomized, Placebo-Controlled Study. Am J Gastroenterol. 2010;105:2218. Available from: /pmc/articles/PMC3180711/
Li S, Yin Y, Xiao D, Zou Y. Supplemental bifid triple viable capsule treatment improves inflammatory response and T cell frequency in ulcerative colitis patients. BMC Gastroenterol. 2021;21:1–12. Available from: https://bmcgastroenterol.biomedcentral.com/articles/https://doi.org/10.1186/s12876-021-01887-2
Xie F, Li S, Fan Y, Li W, Lv Q, Sun X, et al. Efficacy and Safety of Bifidobacterium Quadruple Viable Bacteria Combined with Mesalamine against UC Management: A Systematic Review and Meta-Analysis. Oxid Med Cell Longev. 2022;2022.
Tian C, Huang Y, Wu X, Xu C, Bu H, Wang H. The Efficacy and Safety of Mesalamine and Probiotics in Mild-to-Moderate Ulcerative Colitis: A Systematic Review and Meta-Analysis. Evidence-based Complement Altern Med. 2020;2020.
Belei O, Olariu L, Dobrescu A, Marcovici T, Marginean O. Is It Useful to Administer Probiotics Together With Proton Pump Inhibitors in Children With Gastroesophageal Reflux? J Neurogastroenterol Motil. 2018;24:51. Available from: /pmc/articles/PMC5753903/
Singh G, Haileselassie Y, Briscoe L, Bai L, Patel A, Sanjines E, et al. The effect of gastric acid suppression on probiotic colonization in a double blinded randomized clinical trial. Clin Nutr ESPEN. 2022;47:70–7. Available from: http://clinicalnutritionespen.com/article/S2405457721011098/fulltext
Gwee KA, Goh V, Lima G, Setia S. Coprescribing proton-pump inhibitors with nonsteroidal anti-inflammatory drugs: risks versus benefits. J Pain Res. 2018;11:361. Available from: /pmc/articles/PMC5817415/
Kumar SS, Arvind S, Umpierrez AP. Things We Do for No ReasonTM: Routine use of proton pump inhibitors for peptic ulcer prophylaxis in adults on high-dose corticosteroids. J Hosp Med. 2023;18:630–2. Available from: https://onlinelibrary.wiley.com/doi/full/https://doi.org/10.1002/jhm.13095
Patil JS, Mandave S V, Jadhav SM. Ionotropicaly Crosslinked and Chitosan Reinforced Losartan Potassium Loaded Complex Alginate Beads: Design, Characterization and Evaluation. Malaya J Biosci. 2014;2014:126–33. Available from: www.malayabiosciences.com
Shafagh N, Sabzi M, Afshari MJ. Development of pH-sensitive and antibacterial gelatin/citric acid/Ag nanocomposite hydrogels with potential for biomedical applications. J Polym Res. 2018;25:1–8. Available from: https://link.springer.com/article/https://doi.org/10.1007/s10965-018-1661-9
Bayan MF, Bayan RF. Recent advances in mesalamine colonic delivery systems. Futur J Pharm Sci 2020 61. 2020;6:1–7. Available from: https://fjps.springeropen.com/articles/https://doi.org/10.1186/s43094-020-00057-7
Gul O, Atalar I, Gul LB. Effect of different encapsulating agent combinations on viability of Lactobacillus casei Shirota during storage, in simulated gastrointestinal conditions and dairy dessert. Food Sci Technol Int. 2019;25:608–17. Available from: https://pubmed.ncbi.nlm.nih.gov/31146586/
Mandal S, Hati S, Puniya AK, Khamrui K, Singh K. Enhancement of survival of alginate-encapsulated Lactobacillus casei NCDC 298. J Sci Food Agric. 2014;94:1994–2001. Available from: https://pubmed.ncbi.nlm.nih.gov/24307185/
Cook MT, Tzortzis G, Charalampopoulos D, Khutoryanskiy V V. Production and evaluation of dry alginate-chitosan microcapsules as an enteric delivery vehicle for probiotic bacteria. Biomacromolecules. 2011;12:2834–40. Available from: https://pubmed.ncbi.nlm.nih.gov/21574635/
Smrdel P, Bogataj M, Mrhar A. The Influence of Selected Parameters on the Size and Shape of Alginate Beads Prepared by Ionotropic Gelation. Sci Pharm 2008, Vol 76, Pages 77–90. 2008;76:77–90. Available from: https://www.mdpi.com/2218-0532/76/1/77
Khan MS, Sridhar BK, Srinatha A. Development and Evaluation of pH-Dependent Micro Beads for Colon Targeting. Indian J Pharm Sci. 2010;72:18. Available from: /pmc/articles/PMC2883222/
Narra K, Dhanalekshmi U, Rangaraj G, Raja D, Kumar CS, Reddy PN, et al. Effect of Formulation Variables on Rifampicin Loaded Alginate Beads. Iran J Pharm Res IJPR . 2012;11:715. Available from: /pmc/articles/PMC3813113/
Barbosa JAC, Al-Kauraishi MM, Smith AM, Conway BR, Merchant HA. Achieving gastroresistance without coating: Formulation of capsule shells from enteric polymers. Eur J Pharm Biopharm. 2019;144:174–9. Available from: https://pubmed.ncbi.nlm.nih.gov/31541663/
Kattamuri S, Venkatesan A, Balamurugan SD, Radhakrishnan A, Kuppusamy G, Singh SK. Preparation and Evaluation of Enteric Capsules. Res J Pharm Technol. 2020;13:3603–9. Available from: https://rjptonline.org/AbstractView.aspx?PID=2020-13-8-10
Wu Y, Li S, Jin M, Li D, Zhou Z, Hou H, et al. Preparation of MSZ Hydrogel and Its Treatment of Colitis. Front Pharmacol. 2021;12:2305.
Jaiswal M, Lanjhiyana SK. Fabrication and Evaluations of Dual Crosslinked Mesalamine containing Pectin-Chitosan gel micro beads for controlled and targeted colon delivery. Res J Pharm Technol. 2018;11:4797–804. Available from: https://rjptonline.org/AbstractView.aspx?PID=2018-11-11-4
Rathnam G. Formulation and Evaluation of Colon Targeted Compression Coated Tablet of Mesalamine and Prednisolone for Ulcerative Colitis. Asian J Pharm. 2017;11. Available from: http://www.asiapharmaceutics.info/index.php/ajp/article/view/1407
Jain V, Prasad D, Jain D, Mishra SK, Singh R. Factorial design-based development of measlamine microspheres for colonic delivery. Biomatter. 2011;1:182. Available from: /pmc/articles/PMC3549889/
Badhana S, Garud N, Garud A. Colon specific drug delivery of mesalamine using eudragit S100-coated chitosan microspheres for the treatment of ulcerative colitis. Int Curr Pharm J. 2013;2:42–8. Available from: https://www.banglajol.info/index.php/ICPJ/article/view/13577
Younis MK, Tareq AZ, Kamal IM. Optimization Of Swelling, Drug Loading And Release From Natural Polymer Hydrogels. IOP Conf Ser Mater Sci Eng. 2018;454:012017. Available from: https://iopscience.iop.org/article/https://doi.org/10.1088/1757-899X/454/1/012017
Ceylan O, Karakus H, Cicek H. Design and in vitro antibiofilm activity of propolis diffusion-controlled biopolymers. Biotechnol Appl Biochem. 2021;68:789–800. Available from: https://pubmed.ncbi.nlm.nih.gov/32701174/
Mady FM, Ibrahim S, Abourehab M. Development and evaluation of alginate-gum blend mucoadhesive microspheres for controlled release of Metformin Hydrochloride. J Adv Biomed Pharm Sci. 2021;4:111–8. Available from: https://jabps.journals.ekb.eg/article_159067.html
Sabry SA. Formulation and evaluation of diazepam-loaded intravaginal alginate beads: an investigation study for the treatment of pelvic floor dysfunction. Asian J Pharm Clin Res. 2018;11:97–104. Available from: https://innovareacademics.in/journals/index.php/ajpcr/article/view/25213
Chuong MC, Christensen JM, Ayres JW. New dissolution method for mesalamine tablets and capsules. Dissolution Technol. 2008;15:7–14.
Deshmukh R, Harwansh RK. Preformulation Considerations Development and Evaluation of Mesalamine Loaded Polysaccharide-Based Complex Mucoadhesive Beads for Colon Targeting. Indian J Pharm Educ Res. 2021;55:95–106.
Erdélyi L, Fenyvesi F, Gál B, Haimhoffer Á, Vasvári G, Budai I, et al. Investigation of the Role and Effectiveness of Chitosan Coating on Probiotic Microcapsules. Polymers (Basel). 2022;14. Available from: https://pubmed.ncbi.nlm.nih.gov/35566837/
Solanki D, Motiwale M. Studies on Drug Release Kinetics and Mechanism from Sustained Release Matrix Tablets of Isoniazid using Natural Polymer Obtained from Dioscorea Alata. Int J ChemTech Res. 2020;13:166–73. Available from: https://doi.org/10.20902/IJCTR.2019.130313
Mathematical models of drug release. Strateg to Modify Drug Release from Pharm Syst. 2015;63–86.
Siepmann J, Siepmann F. Mathematical modeling of drug delivery. Int J Pharm. 2008;364:328–43.
Dash S, Murthy PN, Nath L, Chowdhury P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm. 2010;67:217–23. Available from: https://europepmc.org/article/med/20524422
Diaz DA, Colgan ST, Langer CS, Bandi NT, Likar MD, Van Alstine L. Dissolution Similarity Requirements: How Similar or Dissimilar Are the Global Regulatory Expectations? AAPS J. 2016;18:15. Available from: /pmc/articles/PMC4706290/
Thai H, Thuy Nguyen C, Thi Thach L, Thi Tran M, Duc Mai H, Thi Thu Nguyen T, et al. Characterization of chitosan/alginate/lovastatin nanoparticles and investigation of their toxic effects in vitro and in vivo. Sci Reports 2020 101. 2020;10:1–15. Available from: https://www.nature.com/articles/s41598-020-57666-8
Mondéjar-López M, Rubio-Moraga A, López-Jimenez AJ, García Martínez JC, Ahrazem O, Gómez-Gómez L, et al. Chitosan nanoparticles loaded with garlic essential oil: A new alternative to tebuconazole as seed dressing agent. Carbohydr Polym. 2022;277:118815.
Tummala S, Satish Kumar MN, Prakash A. Formulation and characterization of 5-Fluorouracil enteric coated nanoparticles for sustained and localized release in treating colorectal cancer. Saudi Pharm J. 2015;23:308–14.
Teja SB, Shashank PP, Ganesh S, Sarsvatkumar P, Kumar BA. Journal of Excipients and Food Chemicals. J Excipients Food Chem. 2013;4:70–94.
Shi Q, Moinuddin SM, Cai T. Advances in coamorphous drug delivery systems $. Acta Pharm Sin B. 2019;9:19–35. Available from: www.elsevier.com/locate/apsbwww.sciencedirect.com
Mady FM, Ibrahim SR, Abourehab MA, Adv Biomed J, Sci P. Development and Evaluation of Alginate-gum Blend Mucoadhesive Microspheres for Controlled Release of Metformin Hydrochloride. J Adv Biomed Pharm Sci J Homepage. 2021;4:111–8. Available from: http://jabps.journals.ekb.eg
Heikal EJ, Kaoud RM, Gad S, Mokhtar HI, Alattar A, Alshaman R, et al. Development of Novel pH-Sensitive Eudragit Coated Beads Containing Curcumin-Mesalamine Combination for Colon-Specific Drug Delivery. Gels. 2023;9:264. Available from: https://www.mdpi.com/2310-2861/9/4/264/htm
Espíndola SP, Norder B, Koper GJM, Picken SJ. The Glass Transition Temperature of Heterogeneous Biopolymer Systems. Biomacromolecules. 2023;24:1627–37. Available from: https://pubs.acs.org/doi/full/https://doi.org/10.1021/acs.biomac.2c01356
Nyamweya NN. Applications of polymer blends in drug delivery. Futur J Pharm Sci 2021 71. 2021;7:1–15. Available from: https://fjps.springeropen.com/articles/https://doi.org/10.1186/s43094-020-00167-2
Jahan ST, Sadat SMA, Islam MS, Jalil R ul, Chowdhury JA. Effect of Various Excipients on Theophylline-loaded Alginate Beads Prepared by Ionic Cross Linking Technique. Dhaka Univ J Pharm Sci. 2010;9:15–22. Available from: https://www.banglajol.info/index.php/JPharma/article/view/7425
Teixeira AS, Deladino L, Fernandes CF, Martino M, Molina-García AD. Application of Chitosan Coating on Alginate Beads for Cryopreservation Uses. J Chitin Chitosan Sci. 2014;2:46–54.
da Pereira AS, Diniz MM, De Jong G, Gama Filho HS, dos Anjos MJ, Finotelli PV, et al. Chitosan-alginate beads as encapsulating agents for Yarrowia lipolytica lipase: Morphological, physico-chemical and kinetic characteristics. Int J Biol Macromol. 2019;139:621–30.
Hu Y, Peng J, Ke L, Zhao D, Zhao H, Xiao X. Alginate/carboxymethyl chitosan composite gel beads for oral drug delivery. J Polym Res. 2016;23:1–10. Available from: https://link.springer.com/article/https://doi.org/10.1007/s10965-016-1022-5
Aldawsari MF, Ahmed MM, Fatima F, Anwer MK, Katakam P, Khan A. Development and Characterization of Calcium-Alginate Beads of Apigenin: In Vitro Antitumor, Antibacterial, and Antioxidant Activities. Mar Drugs 2021, Vol 19, Page 467. 2021;19:467. Available from: https://www.mdpi.com/1660-3397/19/8/467/htm
Zhang H, Gu Z, Li W, Guo L, Wang L, Guo L, et al. pH-sensitive O-carboxymethyl chitosan/sodium alginate nanohydrogel for enhanced oral delivery of insulin. Int J Biol Macromol. 2022;223:433–45. Available from: https://pubmed.ncbi.nlm.nih.gov/36347366/
Patel N, Lalwani D, Gollmer S, Injeti E, Sari Y, Nesamony J. Development and evaluation of a calcium alginate based oral ceftriaxone sodium formulation. Prog Biomater. 2016;5:117–33. Available from: https://link.springer.com/article/https://doi.org/10.1007/s40204-016-0051-9
Segale L, Giovannelli L, Mannina P, Pattarino F. Calcium Alginate and Calcium Alginate-Chitosan Beads Containing Celecoxib Solubilized in a Self-Emulsifying Phase. Scientifica (Cairo). 2016;2016. Available from: /pmc/articles/PMC4834166/
Kyzioł A, Mazgała A, Michna J, Regiel-Futyra A, Sebastian V. Preparation and characterization of alginate/chitosan formulations for ciprofloxacin-controlled delivery. https://doi.org/10.1177/0885328217714352. 2017;32:162–74. Available from: https://journals.sagepub.com/doi/https://doi.org/10.1177/0885328217714352
Ways TMM, Lau WM, Khutoryanskiy V V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers (Basel). 2018;10. Available from: /pmc/articles/PMC6414903/
Kumari N, Aggarwal G, Harikumar SL. MUCOADHESIVE MICROSPHERES: A REVIEW. J Drug Deliv Ther. 2014;4:48–54. Available from: http://jddtonline.info/index.php/jddt/article/view/953
Notario-Pérez F, Galante J, Martín-Illana A, Cazorla-Luna R, Sarmento B, Ruiz-Caro R, et al. Development of pH-sensitive vaginal films based on methacrylate copolymers for topical HIV-1 pre-exposure prophylaxis. Acta Biomater. 2021;121:316–27.
Mesalamine Delayed-Release Tablets Type of Posting Posting Date Official Date Expert Committee Reason for Revision. Revis Bull. 2019;1–3. Available from: https://www.uspnf.com/sites/default/files/usp_pdf/EN/USPNF/revisions/mesalamine-drt-rb-notice-20191122.pdf
Rodrigues FJ, Cedran MF, Bicas JL, Sato HH. Encapsulated probiotic cells: Relevant techniques, natural sources as encapsulating materials and food applications – A narrative review. Food Res Int. 2020;137:109682.
Severino P, da Silva CF, Andrade LN, de Lima Oliveira D, Campos J, Souto EB. Alginate Nanoparticles for Drug Delivery and Targeting. Curr Pharm Des. 2019;25:1312–34. Available from: https://pubmed.ncbi.nlm.nih.gov/31465282/
Omwancha WS, Mallipeddi R, Valle BL, Neau SH. Chitosan as a Pore Former in Coated Beads for Colon Specific Drug Delivery of 5-ASA. Int J Pharm. 2013;441:343. Available from: /pmc/articles/PMC3556201/
Singh B, Rohit S. Tailoring and evaluating poly(vinyl sulfonic acid)-sterculia gum network hydrogel for biomedical applications. Materialia. 2022;25:101524.
Permanadewi I, Kumoro AC, Wardhani DH, Aryanti N. Modelling of controlled drug release in gastrointestinal tract simulation. J Phys Conf Ser. 2019;1295:012063. Available from: https://iopscience.iop.org/article/https://doi.org/10.1088/1742-6596/1295/1/012063
Palumbo VD, Romeo M, Gammazza AM, Carini F, Damiani P, Damiano G, et al. The long-term effects of probiotics in the therapy of ulcerative colitis: A clinical study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2016;160:372–7. Available from: https://pubmed.ncbi.nlm.nih.gov/27623957/
Arepally D, Reddy RS, Goswami TK. Studies on survivability, storage stability of encapsulated spray dried probiotic powder. Curr Res Food Sci. 2020;3:235. Available from: /pmc/articles/PMC7575842/
Grimaudo MA, Pescina S, Padula C, Santi P, Concheiro A, Alvarez-Lorenzo C, et al. Poloxamer 407/TPGS Mixed Micelles as Promising Carriers for Cyclosporine Ocular Delivery. Mol Pharm. 2018;15:571–84. Available from: https://pubs.acs.org/doi/abs/https://doi.org/10.1021/acs.molpharmaceut.7b00939
Repka MA, McGinity JW. Influence of Vitamin E TPGS on the properties of hydrophilic films produced by hot-melt extrusion. Int J Pharm. 2000;202:63–70. Available from: https://pubmed.ncbi.nlm.nih.gov/10915927/
Teixeira SC, Silva RRA, de Oliveira TV, Stringheta PC, Pinto MRMR, de Soares NFF. Glycerol and triethyl citrate plasticizer effects on molecular, thermal, mechanical, and barrier properties of cellulose acetate films. Food Biosci. 2021;42:101202.
Acknowledgements
The authors would like to thank the Faculty of Pharmaceutical Sciences, PCTE Group of Institutes, Ludhiana, Punjab, India for their continuous support. The authors are thankful to BEC Chemicals Ltd., Mumbai, and Evonik Industries, Mumbai for providing the gift samples of mesalamine and Eudragit® S100 respectively. The authors are thankful to Consern Pharmaceuticals, Ludhiana, Punjab, India, for providing the laboratory and instrumental support.
Funding
This study was funded and supported by the Faculty of Pharmaceutical Sciences, PCTE Group of Institutes, Ludhiana, Punjab, India.
Author information
Authors and Affiliations
Contributions
Jagtar Singh: Designed the study, conceptualization and methodology; Performed the experiments; Analysed and interpreted the data; and prepared the original draft.
Mohit Sharma: Performed the experiments, Analysed and interpreted the data.
Harmeet Singh: Supervised the study; Performed the experiments; and Analysed and interpreted the data.
Pinky Arora: Statistical analysis and interpreted the data.
Puneet Utreja: Contributed reagents; materials, tools, analytical support; and lab infrastructure.
Shubham Kumar: Supervised the study; Analysed and interpreted the data; and prepared the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, J., Sharma, M., Singh, H. et al. Formulation, Characterization and In Vitro Evaluation of Mesalamine and Bifidobacterium bifidum Loaded Hydrogel Beads in Capsule System for Colon Targeted Delivery. AAPS PharmSciTech 25, 61 (2024). https://doi.org/10.1208/s12249-024-02764-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-024-02764-3