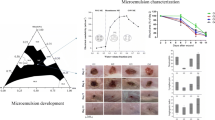
Abstract
Modern drug carrier technologies, such as microemulsions with small droplet sizes and high surface areas, improve the ability of low water solubility active ingredients to permeate and localize. The goal of this study was to create microemulsion formulations for wound healing that contained both fusidic acid (FA), an antibacterial agent, and benzocaine (BNZ), a local anesthetic. Studies on characterization were carried out, including viscosity, droplet size, and zeta potential. The drug-loaded microemulsion had a stable structure with –3.014 ± 1.265 mV of zeta potential and 19.388 ± 0.480 nm of droplet size. In both in vitro release and ex vivo permeability studies, the microemulsion was compared with Fucidin cream and oily BNZ solution. According to the drug release studies, BNZ release from the microemulsion and the BNZ solution showed a similar profile (p > 0.05), while FA release from the microemulsion had a higher drug release compared to Fucidin cream (p < 0.001). The microemulsion presented lower drug permeation (p > 0.05) for both active ingredients, on the other hand, provided higher drug accumulation compared to the control preparations. Moreover, according to the results of in vitro wound healing activity, the microemulsion indicated a dose-dependent wound healing potential with the highest wound healing activity at the highest concentrations. To the best of our knowledge, this developed BNZ- and FA-loaded microemulsion would be a promising candidate to create new opportunities for wound healing thanks to present the active ingredients, which have low water solubility, in a single formulation and achieved higher accumulation than control preparations.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Active components called local anesthetics block the flow of sodium ions and the transmission of nerve impulses, therefore reducing pain [1]. Benzocaine (BNZ), which has poor water solubility, is one of the most widely used local anesthetics [2]. Because of its brief duration of analgesia, BNZ may have systemic adverse effects, including the production of methemoglobin at its high plasma concentrations [3]. BNZ (1–20%) is topically applied to the skin or mucosa to reduce the feeling of pain in the treatment of dental infections, wounds, and burns in many dosage forms, including cream, ointment, gel, solution, and spray [4,5,6,7,8]. However, the onset of action of local anesthetics such as BNZ varies between 30 min and 2 h [5]. Novel drug carrier systems are needed to shorten the onset of action and improve their effectiveness.
Fusidic acid (FA), synthesized by a fungus called Fusidium coccineum, is a low water-soluble steroidal antibiotic used orally, topically, and parenterally [9, 10]. FA, which is effective against strains such as Gram-positive Cocci, Staphylococcus aureus, and Staphylococcus epidermidis inhibits bacterial protein synthesis by preventing the polymerization of terminal amino acid [11]. FA’s broad systemic distribution and absorption to numerous body organs as a result of oral and parenteral treatment cause a number of adverse effects, including rhabdomyolysis, diarrhea, and hepatotoxicity, which decreases the drug’s effectiveness on the skin [12]. Conversely, topical administration of FA was reported to ensure positive results for the treatment of wound [13]. To this end, in recent years, many drug carrier systems of FA such as in situ gel [14], microemulsion [15], liposome [11], and nanocrystal [16] were developed for use in the topical wound treatment.
Wounds that cause deterioration in the structure and function of the skin for various reasons negatively affect the social and financial lives of millions of people in the form of acute and chronic wounds [17, 18]. Acute wounds arise in the form of superficial damage due to a surgical incision, abrasion-related trauma, burns, etc., while chronic wounds form because of the triggering of physiological diseases such as diabetes. Chronic wounds create a more favorable environment for bacterial infections. On the other hand, failure to treat acute wounds quickly and properly leads to chronic wounds [19]. For this reason, effective treatment of wounds critically affects the duration of treatment and patient compliance. In the pharmacological treatment of wounds, active ingredients with different pharmacological effects such as antimicrobials, antibiotics, analgesics, and anesthetics are used topically either alone or in combination to reduce pain and prevent infection [20,21,22,23,24].
Topical delivery is the process of administering a medication to the targeted area of the skin to provide a local effect [25]. Semi-solid preparations are used for this purpose; however, due to the complex structure of the skin, these conventional drug delivery systems may be insufficient, especially for the administration of hydrophobic substances to the skin [19]. In this respect, microemulsions, which are one of the modern drug carrier systems, become very attractive in recent years [26, 27]. Microemulsion, first described by Hoar and Schulman in the 1940s, is thermodynamically stable and single optically isotropic systems [28]. They come to the forefront with their advantages such as being easily prepared by titration method using oil, water, surfactant, and cosurfactant, and having a low viscosity and a high drug permeability [29, 30]. Microemulsions not only increase the penetration ability of the drug but also allow the drug to enter the skin/tissue more quickly [31, 32] They help lessen the systemic negative effects of medications thanks to their ability to be localized in the tissue [33]. Moreover, microemulsions offer an important opportunity for the topical application of active ingredients with a low water solubility [34]. Thanks to these unique advantages, the application of microemulsions containing active ingredients presented very effective results for wound healing [13, 15, 20, 35].
This study aimed to develop a formulation in which BNZ and FA can be presented together in the treatment of superficial wounds. The combination of these two active substances in a conventional semi-solid formulation may cause significant difficulties due to their poor water solubilities and different physicochemical characteristics. By overcoming these difficulties, thanks to an ideal microemulsion with a small droplet size containing oil, water, surfactant, and cosurfactant substances, it is possible to increase the solubility and improve the effectiveness of the active substances without significantly changing the characteristics of the formulation. In this project, due to the advantages of microemulsions over conventional semi-solid formulations and their unique advantages, BNZ- and FA-loaded microemulsion was prepared. Studies were available in the literature, including our earlier work on the creation of FA and BNZ microemulsions [15, 36, 37]. However, there was no research or commercial product including these two active ingredients simultaneously. The main goal of this formulation was to obtain a combined pharmacological effect and localize in the tissue for the treatment of superficial skin injuries such as abrasion and superficial wounds such as mild types of diabetic wounds by providing the desired effectiveness of both faster and for a longer time. Thus, it is possible to achieve both antibacterial and anesthetic effects together by the microemulsion containing FA and BNZ. This will result in both quicker healing and less discomfort from a single formulation, increasing patient compliance while also enhancing the medications’ efficacy through the use of microemulsion.
Materials and Methods
Materials
BNZ (> 98%) was obtained from Acros Organics (USA). FA was kindly gifted from Berko İlaç (Turkey). Ethyl oleate, Cremophor EL, ethanol, propylene glycol, glacial acetic acid, acetonitrile, streptomycin solution (10.000 µg/mL), 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT), and LPS (lipopolysaccharide from Escherichia coli O111:B4) were bought from Sigma (USA). Fucidin cream was bought from Abdi İbrahim İlaç, Türkiye. Before HPLC analysis, the samples were filtered using a membrane filter (0.22 µm, cellulose acetate (CA), Isolab, Germany). L929 cell line was obtained from the American Type Culture Collection (ATCC, USA). Fetal bovine serum (FBS) and Dulbecco’s Modified Eagle’s Medium (DMEM) were supplemented from Gibco, NY, USA.
Solubility Studies
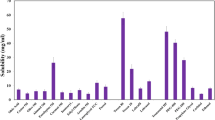
The solubility of BNZ and FA in ethanol, acetonitrile, ethyl oleate, propylene glycol, distilled water, and acetic acid (0.1%) solvents were evaluated separately for each active ingredient. To this end, first, 1 mL of solvent was added into a microcentrifuge tube, and a large amount of active ingredient was transferred to the tube. The mixture was shacked in a horizontal shaker mixer (SSL2, Stuart, UK) for 24 h; if there was no precipitate at the end of the period, some more active ingredients were added, and the same procedures were repeated. Subsequently, it was centrifuged in a refrigerated centrifuge (3–18 KS, Sigma, Germany) at + 4°C and 14,000 rpm for 30 min. A sample of 0.5 mL was taken from the supernatant and diluted using a mobile phase. The solution was filtered through a membrane filter (CA, Ø = 25 mm) and analyzed by an HPLC device (Agilent 1100, USA).
Preparation of Microemulsions
The formulations were prepared using ethyl oleate as an oil phase, Cremophor EL as a surfactant, and ethanol and propylene glycol (1:1) as co-surfactants. In each phase diagram created to determine the ideal microemulsion, the ratios of the surfactant to cosurfactant were varied as 1:1, 2:1, 3:1, 4:1, 5:1, 1:2, 1:3, and 1:4 (w/w). These mixtures were titrated, drop-by-drop, with distilled water while being stirred at 300 rpm and ambient temperature. After the mixtures were equilibrated, their appearance was visually evaluated. After the determination of the microemulsion region in the pseudo-ternary phase diagrams created using a software program, the lead microemulsions were selected at the required component ratios according to the center of the region [38]. The microemulsion was loaded with 2% of BNZ and 2% of FA.
Characterization of Microemulsions
The physicochemical characterization of generated microemulsions, including those on droplet size, polydispersity index (PDI), zeta potential, refractive index, electrical conductivity, pH, and viscosity was assessed to determine whether they were suitable for topical application. For each sample, the experiments were run five times at ambient temperature.
The dynamic light scattering method was used to measure the average droplet size and PDI of the microemulsions (Nano ZS, Malvern Instruments, United Kingdom). The data for particle size and PDI were calculated by averaging ten measurements made with disposable cells at 173°.
Using disposable flat-folded capillary zeta cells, the zeta potential was measured (Nano ZS, Malvern Zetasizer, UK). The Helmholtz-Smoluchowski equation was used to compute the zeta potential resulting from electrophoretic mobility in an electric field of 40 V/cm. The software took care of the procedure. A viscometer was used to measure the formulations’ viscosities (SV-10, AND Vibro Viscometer, Japan). A digital pH meter was used to determine the formulations’ pH levels (Mettler Toledo, Switzerland). Using a refractometer, the compositions’ refractive index values were assessed (RX-7000 CX, Atago, Japan). To identify the kind of microemulsion, the electrical conductivity of the microemulsions was measured (Nano ZS, Malvern Zetasizer, UK).
Physical Stability
The physical stability of the formulations was evaluated under temperature and centrifugation conditions. The microemulsions were centrifuged at 5000 rpm and ambient temperature for 30 min to assess whether there was any precipitation or phase separation [39]. In addition, the microemulsions were subjected to 6 cycles of cooling-heating conditions at 5 ± 1°C and 45 ± 2°C. The microemulsion, which was kept at 5 ± 1°C in a refrigerator for 24 h, was then kept in an oven (MMM Medcenter, Ecocell, Germany) at 45 ± 2°C for 24 h. Physical changes in the microemulsion were visually assessed at the end of 6 cycles [40].
Drug Content Uniformity
To determine the content uniformity of the microemulsion, 0.5 g of microemulsion was weighed in a beaker, and 100 mL of acetonitrile was transferred to the beaker. The mixture was stirred until the microemulsion was completely dispersed. After that, a membrane filter was used to filter 1 mL of the aliquot. The procedures were repeated in triplicate for each batch, and each sample was analyzed at 210 nm by the HPLC device.
For the quantitative determination of BNZ and FA, a quantification method was developed and validated using the HPLC equipped with a UV detector. A C18 column (InertSustain C18, 150 × 4.6 mm, 5 μm, GL Sciences, Japan) was utilized for the analysis. The mobile phase consisted of 0.1% acetic acid and acetonitrile (v/v, 30:70). While the flow rate was 1 mL, the injection volume was 20 µL. The column temperature was determined as 25°C and the wavelength was 210 nm. Precision, repeatability, reproducibility, stability, selectivity, limit of detection (LOD), and limit of quantification (LOQ) analyses were performed to validate the developed method [41].
In Vitro Drug Release
Drug release studies of the microemulsions were evaluated by a dialysis bag method [42]. Fucidin cream (Abdi İbrahim, Turkey), which was a commercial product of FA, and an oily BNZ solution were used to compare the drug release profiles of the microemulsion. The oily BNZ solution (2%, w/w) was prepared by dissolving BNZ in ethyl oleate. Accurately weighed 1 g of the microemulsion, the BNZ solution, and the cream were individually transferred to dialysis bags (molecular weight cut off 12–14,000 kDa, SpectraPor, Spectrum Labs, Germany). Magnetic closure clips were used for closing the mouths of the bags. The studies were performed at 50 rpm and 32 ± 1°C. Phosphate buffer (pH 7.4) and ethanol (v/v, 70:30) were used as a release medium to ensure sink condition. After placing the dialysis bags into beakers containing 200 mL of the medium, 1 mL of the sample was taken at specified times (0.5 to 24 h) and filtered using the membrane filter (CA, Ø = 25 mm). As soon as the sample was taken, the medium was completed with the fresh medium. The studies were performed with four replicates for each batch, and the samples were analyzed by the HPLC device.
The release profiles of BNZ and FA from the microemulsion were compared using the similarity factor (f2) against the BNZ solution (2%) and Fucidin cream, respectively. In addition, kinetic analyses were performed to elucidate the release profiles of the active ingredients. To this end, zero-order (Eq. 1), first-order (Eq. 2), Higuchi (Eq. 3), Hixson-Crowell (Eq. 4) kinetics, and Korsmeyer-Peppas (Eq. 5) models were applied. Here, Mt and M∞ denoted the percentage or concentration of drug release at “t” and equilibrium time, respectively. k0, k1, kH, and kHC represented the constants of zero order, first order, Higuchi, and Hixson-Crowell release kinetics, respectively. Also, kKP and n symbolized the constant of the geometric and structural character, and the constant of the release mechanism of the Korsmeyer-Peppas model, respectively [43].
Ex vivo Research on Permeability and Penetration
The National Institutes of Health (NIH) rules were followed for conducting the ex vivo study. In compliance with Istanbul Medipol University Ethical Council regulations (approval no: 12.06.2023–38), shaved skins harvested from Balb/c mice were used. The microemulsion’s permeability was evaluated by comparing it with the BNZ solution (2%) and Fucidin cream (Abdi İbrahim İlaç, Turkey). The experiments were performed using a Franz diffusion cell system (8 mL of receptor volume, 1.00 cm2 of diffusion area, FDC-6 T series transdermal system, Logan, USA) at 50 rpm for 24 h. After filling the receptor compartment with phosphate buffer (pH 7.4): ethanol (70:30), skin from Balb/c mice was positioned between the donor and receptor compartments and secured with a metal clamp. To ensure that the skin surface was 32°C, the receptor chamber was mixed at 37 ± 0.5°C [42, 44]. Accurately weighed 1 g of the microemulsion, the cream, and the solution were individually transferred onto the skins. At the specified time intervals (0.5 to 24 h), a sample (0.5 mL) was taken, and the receptor volume was completed with the fresh medium. The samples were filtered (CA, Ø = 13 mm) and analyzed with the HPLC. The studies were carried out in triplicate. At the end of 24 h, the amounts of the active ingredients accumulated in the skin were determined. First, the skin was washed with distilled water, then cut into small pieces, taken into a tube containing ethanol, and finally vortexed for 20 min. At the end of the process, the tubes were centrifuged at 4°C and 5000 rpm for 30 min. The supernatant was sampled and filtered, then analyzed by the HPLC.
Cytotoxicity Assay
The cytotoxicity experiments for formulation were conducted using healthy mouse fibroblast cell line L929 which was grown in DMEM supplemented with 10% FBS (Gibco, NY, USA), 1% penicillin (10,000 units/mL), and streptomycin (10,000 mg/mL) in an incubator that provides the cell cultures with 5% CO2 at 37°C. L929 cells were seeded in a 96-well plate in the final concentration of 1 × 105 cells/mL and incubated for 24 h. Then, the cells were treated with different doses of the substances (0.125–0.25–0.5–1 mg/mL). Following 24 h of incubation, the medium was discarded, and 100 µL of MTT solution (500 µg/mL) was applied to each well. Post application, the cells were incubated for another 2 h. Then, the MTT solution was discarded, and formazan crystals were dissolved by using 100 µL of isopropanol [45]. Doses that indicated viability lower than 70% when compared with the medium control group (M) were considered cytotoxic. The optical density to determine the cell viability was analyzed at 570 nm by an ELISA microplate reader (Thermo Multiskan Spectrum, Finland) and calculated with Eq. 6:
In Vitro Wound Healing Assay
The scratch wound healing assay was carried out on a 24-well-plate seeded with L929 cell line with a concentration of 2 × 106 cells/mL in culture media DMEM supplemented with 10% FBS and 1% Pen-Strep. The plate was marked with three parallel lines that were evenly spaced from one another before seeding to give a site for photographs of the wounds to be taken at various points throughout the experiment. Then, the cells were incubated for 24 h to maintain a 100% confluence. Subsequently, to replicate a wound in the cultured tissue, the cells were scratched using a cell scratcher (SPL, Life Sciences, South Korea). The wells were then washed twice with phosphate-buffered saline (PBS), and 500 µL of fresh media was added to each well. The wound images of hour 0 were taken using a microscope at 10 × (AxioCam, Zeiss, Germany). The blank and drug-loaded formulations were separately applied to the cells in a dose that was not cytotoxic, and the medium group (M) was considered as a control. The wound images were taken at different times of the experiment to determine the wound closure. The images that were obtained in the 0th (t0) and 24th (tfinal) hours of the experiment were analyzed by ImageJ analysis software (NCBI, USA) by measuring the width of the wound [46]. The reduction of the size of the wound area is calculated by using the formula below (Eq. 7):
Statistical Analyses
All values were shown as mean and standard deviation (SD). GraphPad Prism Software (Version 9.0.1., CA, USA) was used to conduct the statistical analysis. Student’s t test was used to statistically evaluate the data. p < 0.05 was considered statistically significant.
Results and Discussion
In this work, the microemulsion formulation containing BNZ with a local anesthetic effect and FA with an antibacterial effect was developed by the titration method. The lead microemulsion was determined by the pseudo-ternary phase diagram. In this section, the findings obtained from characterization studies, in vitro drug release studies, ex vivo penetration and permeability studies, in vitro cytotoxicity, and wound healing activity studies were presented, respectively.
Solubility Studies
The samples were analyzed by the HPLC method to determine the solubility of BNZ and FA in various solvents. In this context, the solubility of the active ingredients in distilled water, ethyl oleate, ethanol, and propylene glycol used in the preparation of microemulsions, and in acetonitrile and acetic acid (0.1%) used as HPLC mobile phase was determined. The results are demonstrated in Table I. The water solubility of BNZ and FA was 0.73 ± 0.04 mg/mL and 0.0037 ± 0.0003 mg/mL, respectively. These values were consistent with the studies in the literature [14, 47]. In addition, both active ingredients had high solubility in ethanol. As a result, it was seen that the components of the microemulsion were in the amount that could provide the solubility of the active ingredients.
Preparation of Drug-Loaded Microemulsion
Although microemulsions can form spontaneously at appropriate temperatures and component ratios, low-energy processes such as mixing or heating are generally applied to facilitate their preparation. The titration method is the most used in the laboratory-scale production of microemulsions. Based on the method, a microemulsion is formed by drop-by-drop addition of the oil or water phase to the main mixture [48]. Blank microemulsions consisting of ethyl oleate, Cremophor EL, ethanol, propylene glycol, and water were prepared by the titration method. After determining the ideal microemulsion, a drug-loaded microemulsion (oil/water) was prepared with the addition of BNZ (2%) and FA (2%), and characterization studies were performed.
The ternary phase diagram showing the ideal microemulsion with a ratio of 1:1 (cosurfactant:surfactant) is presented in Fig. 1, and the component amounts of the content of this formulation are given in Table II. Ethyl oleate, used as the oil phase in this study, is an ester of oleic acid and has the potential to increase the solubility of substances with a low water solubility and expand the microemulsion area [49]. It was also reported that ethyl oleate acted as a penetration enhancer to increase skin permeability [50]. Ethanol and propylene glycol are cosurfactants that enable the formation of microemulsions with both solubility and penetration-enhancing properties [42]. Cremophor EL is a surfactant, compatible with ethyl oleate, and has strong emulsifying properties due to its high content of ethylene oxide. With an HLB value of 12–14, this surfactant can form oil/water (O/W) emulsions [51].
Characterization of Microemulsion
The results of the characterization studies are shown in Table III. The pH of the blank formulation was close to neutral, and the pH of the drug-loaded formulation was found to be approximately 6.4 (p < 0.0001). The addition of BNZ and FA decreased the pH due to the weak acid character of these substances [52, 53]. Skin pH is in a wide range between 4 and 7, and although it is reported in the literature to vary, it is generally accepted as slightly acidic [54]. Also, previous studies have shown that acidic or closer to the skin pH values were more convenient for wound healing [55]. It can be said that the pH of the drug-loaded formulation was close to the skin pH and therefore suitable for wound healing.
Due to the viscosity of the drug-loaded microemulsion being 338.400 ± 2.074 mPa.s, the formulation was suitable for topical application. Incorporation of a high amount of the drugs was found to cause a statistically significant change in the viscosity without affecting the stable structure of the formulation (p < 0.0001). Badawi et al. [56] reported that the addition of increasing concentrations of salicylic acid significantly reduced the viscosity of the microemulsion-based gel.
The conductivities of blank and drug-loaded microemulsions were 0.427 ± 0.015 and 0.386 ± 0.007 mS/cm, respectively. The oil phase is mostly electrolyte-free [57], and approx. 11% of this formulation was oil. According to the obtained results, the conductivity values indicated that the outer phase of the microemulsion was water. The refractive index of blank and drug-loaded formulations was very close to each other and was found to be between approx. 1.42 and 1.43 (p > 0.05). The refractive index is one of the parameters used to evaluate the stability of the formulation. This parameter, which is also evaluated as the degree of refraction of light, generally shows a refractive index (1.333) close to pure water in microemulsions [39, 58, 59]. The results showed that the formulations were clear and transparent, consistent with visual evaluation.
The zeta potential, which is an insight into the stability of a colloidal system, enables the determination of the practical surface charge of a formulation. The zeta potential of the drug-loaded formulation was found to be − 3.014 ± 1.265 mV. The use of non-ionic surfactant reduces the magnitude of the zeta potential as a result of the decrease in the surface charge of the droplets [59]. For the stability of disperse systems, it is aimed that the zeta potential is greater than + 30 mV or less than − 30 mV [60], but thermodynamically stable microemulsions are stable even if the zeta potential is quite close to zero [61,62,63]. In addition, the high negative surface charge (close to zero) means that the formulation is stable, and aggregation will not occur [64]. Similarly, Goindi et al. [65] stated that the fact that the zeta potential of microemulsions is close to zero makes the formulations more stable. Tang et al. [51] showed that the resveratrol microemulsions prepared using Cremophor RH 40 were stable with zeta potential values of -2.18 and − 3.25 mV. Monton et al. [66] stated that the zeta potential values of the microemulsions they developed, ranging from − 1.09 to + 0.21 mV, were caused by Tween 80, a non-ionic surfactant. In another study, the researchers who developed the microemulsion of clopidogrel found the zeta potential of the formulation to be − 6.34 and − 3.02 mV. According to the results of 6-month stability studies, it was observed that microemulsions are stable with no changes in droplet size, transmittance (%), and physical appearance [63]. As a consequence, the formulations were stable when assessed in the context of the literature.
The droplet size of microemulsions is generally aimed to be between 10 and 140 nm [67]. The droplet size of the blank and drug-loaded formulations was 23.076 ± 0.285 and 19.388 ± 0.480 nm, respectively. Therefore, the droplet size of the microemulsions was appropriate. It was determined that drug loading decreased the mean droplet size (p < 0.001). This result supports similar studies that detected that following drug loading, the droplet size reduced [68, 69]. This finding can be related to the functional groups of active substances. Active substances that disperse into the emulsifying layer or oil layer can interact with these components of the microemulsion through hydrogen bonding, resulting in a decrease in the mean droplet size [69]. It was stated that, thanks to this small droplet size obtained, the active ingredients could reach the lower layers of the skin/tissue with an increase in surface area, and thus, their pharmacological effectiveness would be increased with higher absorption. At the same time, the small droplet size resists gravity and contributes to the stability of the formulation [70]. The PDI of the blank and the drug-loaded microemulsion was found to be less than 0.1. PDI, which gives information about droplet size distribution, is graded between 0 and 1 [71]. A PDI value less than 0.1 means that the formulation with a very narrow distribution of droplet size is homogeneous and monodispersed [72]. The obtained findings indicated that the formulation had a homogeneous distribution.
Physical Stability
Microemulsions are thermodynamically stable and spontaneously formed colloidal systems with a long shelf life [73, 74]. The better stability of microemulsions compared to vesicular systems such as conventional liposomes makes them an alternative carrier system for many active ingredients, which are applied topically [75]. The physical stability of microemulsions was evaluated visually by applying stress conditions such as centrifugation and temperature. As a result of the high centrifugal force applied, no sedimentation or flocculation was observed. On the one hand, in the heating–cooling studies, when the blank microemulsion was kept at 5°C, its homogenization began to deteriorate, and a slightly cloudy whiteness began to occur. On the other hand, it was determined that this situation was not seen in the samples stored at 45°C and room temperature, and when the sample stored at 5°C was kept at ambient temperature, this situation disappeared, and the formulation was homogeneous again. No physical changes, sedimentation, flocculation, or phase separation were observed in the drug-loaded microemulsion. This change of the blank microemulsion at 5°C was thought to be due to Cremophor EL. The melting point of Cremophor EL is approx. 19–20°C [76]. The microemulsion was homogeneous at room temperature, but slightly cloudy when refrigerated. However, since such a situation was not observed in the drug-loaded microemulsion, it was stable.
Drug Content Uniformity
The content uniformity results of the drug-loaded microemulsion were 99.40 ± 1.62% and 99.53 ± 0.97% for BNZ and FA, respectively. The microemulsion had high drug loading capacity, and the low standard deviations indicated that they were homogeneous.
In Vitro Drug Release Studies and Release Kinetics
The release characteristics of active ingredients from the developed microemulsion were compared with the BNZ solution and Fucidin cream. Since there was no commercial product containing 2% of BNZ in the market, an oily solution of BNZ was prepared by dissolving BNZ (2%, w/w) in ethyl oleate. The drug release profiles are presented in Fig. 2. According to the findings of the dialysis bag method drug release studies, BNZ was released from the solution and the microemulsion at 98.30 ± 0.65% and 97.71 ± 1.03%, respectively, after 24 h, while FA was released from Fucidin cream and the microemulsion at 19.54 ± 1.89% and 67.83 ± 6.32%, respectively. The FA release from the microemulsion was increasingly higher than Fucidin cream (p < 0.001). In contrast, there was no statistically significant difference in BNZ release between the BNZ solution and the microemulsion (p > 0.05).
The similarity factor (f2) is calculated in the evaluation of the similarity of the release profiles of the drug delivery systems. The release profiles of the active ingredients in the microemulsion and the release profile of BNZ solution (2%) and Fucidin cream were discussed separately in terms of similarity. As a result, f2 < 50 means dissimilar, while f2 > 50 is similar [77]. The similarity of the BNZ release profiles of the microemulsion and the BNZ solution was 58.09 (similar), while the similarity of the release of FA from the microemulsion and Fucidin cream was found to be 36.58 (dissimilar). It was expected that the release of oil-soluble BNZ from an oily solution and the release from the microemulsion would be similar. However, the percentage of release of FA from Fucidin cream after 24 h was found to be quite low. FA release increased by approximately three times as a result of the created microemulsion. Many studies have shown that the drug release of hydrophobic drugs from microemulsions or microemulsion-based gels occurred faster or at higher levels than oil-based semi-solid formulations [78,79,80,81]. At the same time, microemulsions can provide controlled and prolonged release. In a study by Okur et al. [15], a microemulsion-based gel formulation of FA was prepared for the treatment of wound healing. According to the findings of drug release tests, the formulation showed a prolonged release because it was microemulsion-based. Consequently, our findings were compatible with previous research.
The release kinetics of active ingredients from the microemulsion were investigated using model-dependent kinetics. The results are shown in Table IV. The kinetic model with the highest correlation coefficient (r2) obtained from the kinetic equations was accepted as the most appropriate model. While the highest r2 was found with Higuchi kinetic (0.8966) for BNZ, the highest r2 values were obtained using Higuchi (0.9403) and zero-order (0.9893) kinetics for FA. The zero-order kinetic model is defined as the drug release independent of the concentration, while the Higuchi model is expressed as the square root of the time-dependent process based on Fickian diffusion [78, 82]. In the Korsmeyer-Peppas model, the r2 values for BNZ and FA were 0.9993 and 0.9782, respectively, while n values were determined as 0.8170 and 1.0091, respectively. The Korsmeyer-Peppas model is applied for data up to 60% of drug release, and the release mechanism is evaluated with “n value” (diffusion exponent: the slope of the line) [83]. n ≤ 0.5 indicates Fickian diffusion controlled release (non-steady); 0.5 < n < 1.0 is non-Fickian diffusion or anomalous, and n ≥ 1 is case II (zero order) [84, 85]. In non-Fickian diffusion, the release of active ingredients from the formulation happens as a combination of controlled release through erosion and diffusion [86]. According to these results, the release of BNZ from the microemulsion occurred by non-Fickian diffusion, case II transport mechanism. In addition, it was proven by the Korsmeyer-Peppas model that the release of FA from the microemulsion was time-independent.
Ex Vivo Research on Permeability and Penetration
Topical treatment and topical formulations come to the fore in wound treatment compared to systemic treatment [87]. To evaluate the effectiveness of topical formulations (microemulsion, nanoparticle, nanocrystal, gel, etc.) from this point of view, ex vivo research can be carried out using biological membranes, mice, rat, and goat skins [14, 16, 21, 88, 89]. One of the most important advantages of topically applied microemulsions is their good penetration and localization ability into the skin and tissue [74]. Although they penetrate the lower layers of the skin, the entry to the systemic circulation is limited. This is a very valuable advantage for formulations that are desired to have a local effect. In our project, it was aimed that the prepared microemulsion formulation had a local effect. For this purpose, the permeability of the microemulsion using Balb/c mice skin by the Franz diffusion cell system was compared with the commercial product (Fucidin cream) and the oily solution of BNZ. The results of the permeability studies are shown in Fig. 3. According to the results of the studies carried out for 24 h, the solution and the marketed cream showed a cumulative permeability of 6.78% (BNZ) and 1.54% (FA), respectively, while the permeability of the developed microemulsion was found to be 3.57% and 0.28% for BNZ and FA, respectively. At the end of the study, the amount of drug accumulated in the skin was detected after the skin was cut and treated with a suitable solvent. The microemulsion provided approx. 1.6-fold and fourfold greater skin accumulation for BNZ and FA, respectively, compared to the preparations (Fig. 3). These results indicated that the microemulsion could penetrate the skin and tissue better and reach systemic circulation less.
Aksu et al. [14] developed in situ gels of FA for the treatment of burn wounds. According to ex vivo permeation studies, the amount of active ingredient both passing through the skin and accumulating in the skin was found to be less than 1%. These low permeation values were associated with the poor solubility of FA in water, the partition coefficient of FA, and the viscosity of the gel. In another study, Almostafa et al. [90] prepared nanoemulgel of FA using surfactants and penetration-enhancing agents such as Transcutol P and Tween 80. It was found that the developed formulation had penetration-enhancing properties due to its nanoemulsion-based, thus had higher permeability than both the classical gel and FA solution. In our previous study conducted in 2017 [36], it was concluded that BNZ-loaded microemulsion and microemulsion-based gels achieved both higher permeation and penetration values than BNZ solution. The microemulsion and the microemulsion-based gels showed a higher drug accumulation with 9.3% and 5.3–7.9%, respectively. While the microemulsion showed higher permeation ability than the microemulsion-based gel, this result was due to the lower viscosity of the microemulsions, although the formulations contained the same penetration enhancers in the same amount. In another study, microemulsions containing indomethacin and BNZ were simultaneously developed by El Maghraby et al. [4] showed 10- and 7-times higher flux values, respectively, than the solutions of these active ingredients. As a result, it was seen that microemulsion and nanoemulsion-based formulations, which offer advantages such as high surface area, low surface tension, high solubility, and high drug loading capacity, thanks to their small droplet size, would increase the effectiveness of the treatment of wound with the accumulation and permeation of these two drugs, which have low water solubility and poor permeability, and our findings were compatible with the aforementioned literature.
Cytotoxicity Assay
The effect of 24-h exposure to four different doses of the control group, blank, and drug-loaded formulations on L929 cell lines was determined, and the detected viability was above 70%. Therefore, the formulation was considered not cytotoxic (Fig. 4). In vitro cytotoxicity studies indicated that the formulation could be safely used for wound healing.
In Vitro Wound Healing Activity
Wound healing is a quite complex process that comprises cellular changes such as inflammation, angiogenesis, re-epithelialization, and granulation tissue formation. In the beginning stages of wound healing, fibroblasts, the most common cells in skin tissue, actively proliferate and encourage the production of new extracellular matrix and thick actin myofibroblasts [91]. Since the migration and proliferation abilities of fibroblasts play an important role in wound healing, L929 fibroblast cells were used as an in vitro model to investigate the wound healing ability of many substances or formulations [92,93,94]. To this end, the scratch assay, an easy, low-cost, and well-developed method of cell migration to other methods [95], was applied to shed light on in vivo wound healing in this study. Different concentrations of the formulations were used to determine the rate of change in the mitigation of L929 in comparison to the control group consisting of the untreated culture medium (M). The cellular proliferation and mitigation were photographed at 0 and 24 h. The results are shown in Fig. 5 and Table V. The average rates of cellular proliferation of the medium were determined as 78.07 ± 3.21% at the 24th h. In the wound healing assay, the microemulsion indicated a dose-dependent wound healing potential with the highest wound healing activity at the highest concentrations. However, a literature search regarding BNZ as a wound healing agent did not yield significant results. Previous studies on FA indicate that it has been previously used as a treatment for skin infections topically [15]. The combination of FA and BNZ could yield an alternative remedy in approaches to wound healing.
Conclusion
It was successfully obtained BNZ and FA-loaded microemulsion consisting of ethyl oleate, Cremophor EL, propylene glycol, and ethanol with a high emulsion area (684.570). The microemulsion had a stable structure with a low PDI, small droplet size, low zeta potential value, and a viscosity value of 338 mPa.s and was suitable for topical application. In in vitro release studies, BNZ release from the ethyl oleate-based BNZ solution and the microemulsion showed a similar release profile, while the microemulsion achieved a higher release percentage for FA than Fucidin cream. As a result of permeability studies, a higher drug accumulation in the skin was provided for both FA (430.10 ± 147.31 µg/cm2) and BNZ (620.22 ± 166.43 µg/cm2) compared to the control preparations (Fucidin cream-129.67 ± 60.01 µg/cm2 and BNZ solution-391.50 ± 57.28 µg/cm2). Moreover, it was shown with an in vitro wound healing assay that the microemulsion indicated a dose-dependent wound healing potential with the highest wound healing activity at the highest concentrations. The microemulsion containing FA and BNZ, prepared for use in wound healing, is an alternative to oily FA cream, and it is not only antibacterial but the anesthetic effect to be obtained, thanks to BNZ, as a result of decreasing the sensation of pain, will increase patient compliance. Therefore, the healing process is expected to be faster and more effective, thanks to the unique opportunities offered by the microemulsion. Additionally, this study enlightens the combine usage of wound healing active ingredients in a single microemulsion system for treatment.
Data Availability
Data will be made available on request.
References
Mura P, Maestrelli F, González-Rodríguez ML, Michelacci I, Ghelardini C, Rabasco AM. Development, characterization and in vivo evaluation of benzocaine-loaded liposomes. Eur J Pharm Biopharm. 2007;67:86–95. https://doi.org/10.1016/j.ejpb.2007.01.020.
Abd El-Alim SH, Kassem AA, Basha M. Proniosomes as a novel drug carrier system for buccal delivery of benzocaine. J Drug Deliv Sci Technol. 2014;24:452–8. https://doi.org/10.1016/S1773-2247(14)50087-1.
Moraes CM, de Matos AP, de Paula E, Rosa AH, Fraceto LF. Benzocaine loaded biodegradable poly-(d, l-lactide-co-glycolide) nanocapsules: factorial design and characterization. Mater Sci Eng B Solid-State Mater Adv Technol. 2009;165:243–6. https://doi.org/10.1016/j.mseb.2009.06.011.
El Maghraby GM, Arafa MF, Osman MA. Microemulsion for simultaneous transdermal delivery of benzocaine and indomethacin: In vitro and in vivo evaluation. Drug Dev Ind Pharm. 2014;40:1637–44. https://doi.org/10.3109/03639045.2013.841186.
De Araujo DR, Padula C, Cereda CMS, Tófoli GR, Brito RB, De Paula E, et al. Bioadhesive films containing benzocaine: correlation between in vitro permeation and in vivo local anesthetic effect. Pharm Res. 2010;27:1677–86. https://doi.org/10.1007/s11095-010-0151-5.
Vohra R, Huntington S, Koike J, Le K, Geller RJ. Pediatric exposures to topical benzocaine preparationsreported to a statewide poison control system. West J Emerg Med. 2017;18:923–7. https://doi.org/10.5811/westjem.2017.6.33665.
Al-Melh MA, Andersson L. Comparison of topical anesthetics (EMLA/Oraqix vs benzocaine) on pain experienced during palatal needle injection. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontology. 2007;103:16–20. https://doi.org/10.1016/j.tripleo.2006.11.033.
Aronson JK. Meyler’s side effects of drugs : the international encyclopedia of adverse drug reactions and interactions. 16th ed. Amsterdam: Elsevier; 2016.
Cé R, Pacheco BZ, Ciocheta TM, Barbosa FS, Alves A de CS, Dallemole DR, et al. Antibacterial activity against Gram-positive bacteria using fusidic acid-loaded lipid-core nanocapsules. React Funct Polym. 2021;162. https://doi.org/10.1016/j.reactfunctpolym.2021.104876.
Rençber S, Özcan Bülbül E, Üstündağ Okur N, Ay Şenyiğit Z. Preparation and detailed characterization of fusidic acid loaded in situ gel formulations for ophthalmic application. J Res Pharm. 2021;25:1–12. https://doi.org/10.35333/jrp.2021.291.
Nicolosi D, Cupri S, Genovese C, Tempera G, Mattina R, Pignatello R. Nanotechnology approaches for antibacterial drug delivery: preparation and microbiological evaluation of fusogenic liposomes carrying fusidic acid. Int J Antimicrob Agents. 2015;45:622–6. https://doi.org/10.1016/j.ijantimicag.2015.01.016.
Wadhwa S, Singh B, Sharma G, Raza K, Katare OP. Liposomal fusidic acid as a potential delivery system: a new paradigm in the treatment of chronic plaque psoriasis. Drug Deliv. 2016;23:1204–13. https://doi.org/10.3109/10717544.2015.1110845.
Jyoti K, Malik G, Chaudhary M, Sharma M, Goswami M, Katare OP, et al. Chitosan and phospholipid assisted topical fusidic acid drug delivery in burn wound: strategies to conquer pharmaceutical and clinical challenges, opportunities and future panorama. Int J Biol Macromol. 2020;161:325–35. https://doi.org/10.1016/j.ijbiomac.2020.05.230.
Aksu NB, Yozgatlı V, Okur ME, Ayla Ş, Yoltaş A, Üstündağ ON. Preparation and evaluation of QbD based fusidic acid loaded in situ gel formulations for burn wound treatment. J Drug Deliv Sci Technol. 2019;52:110–21. https://doi.org/10.1016/j.jddst.2019.04.015.
Okur ME, Ayla Ş, Yozgatlı V, Aksu NB, Yoltaş A, Orak D, et al. Evaluation of burn wound healing activity of novel fusidic acid loaded microemulsion based gel in male Wistar albino rats. Saudi Pharm J. 2020;28:338–48. https://doi.org/10.1016/j.jsps.2020.01.015.
Ahmed IS, Elnahas OS, Assar NH, Gad AM, Hosary R El. Nanocrystals of fusidic acid for dual enhancement of dermal delivery and antibacterial activity: ın vitro, ex vivo and in vivo evaluation. Pharmaceutics. 2020;12. https://doi.org/10.3390/pharmaceutics12030199.
Pekmez M, Milat NS. Evaluation of in vitro wound healing activity of thymoquinone. Eur J Biol. 2020;79:151–6. https://doi.org/10.26650/EurJBiol.2020.0044.
Stan D, Tanase C, Avram M, Apetrei R, Mincu NB, Mateescu AL, et al. Wound healing applications of creams and “smart” hydrogels. Exp Dermatol. 2021;30:1218–32. https://doi.org/10.1111/exd.14396.
Gwarzo ID, Mohd Bohari SP, Abdul Wahab R, Zia A. Recent advances and future prospects in topical creams from medicinal plants to expedite wound healing: a review. Biotechnol Biotechnol Equip. 2022;36:81–93. https://doi.org/10.1080/13102818.2022.2053340.
Ryu KA, Park PJ, Kim SB, Bin BH, Jang DJ, Kim ST. Topical delivery of coenzyme Q10-loaded microemulsion for skin regeneration. Pharmaceutics. 2020;12:1–15. https://doi.org/10.3390/pharmaceutics12040332.
Üstündağ Okur N, Hökenek N, Okur ME, Ayla Ş, Yoltaş A, Siafaka PI, et al. An alternative approach to wound healing field; new composite films from natural polymers for mupirocin dermal delivery. Saudi Pharm J. 2019;27:738–52. https://doi.org/10.1016/j.jsps.2019.04.010.
Kesici S, Kesici U, Ulusoy H, Erturkuner P, Turkmen A, Arda O. Effects of local anesthetics on wound healing. Brazilian J Anesthesiol. 2018;68. https://doi.org/10.1016/j.bjan.2018.01.016.
Purcell A, Buckley T, King J, Moyle W, Marshall AP. Topical analgesic and local anesthetic agents for pain associated with chronic leg ulcers: a systematic review. Adv Ski Wound Care. 2020;33:240–51. https://doi.org/10.1097/01.ASW.0000658572.14692.fb.
Thapa RK, Diep DB, Tønnesen HH. Topical antimicrobial peptide formulations for wound healing: current developments and future prospects. Acta Biomater. 2020;103:52–67. https://doi.org/10.1016/j.actbio.2019.12.025.
Brown MB, Martin GP, Jones SA, Akomeah FK. Dermal and transdermal drug delivery systems: current and future prospects. Drug Deliv J Deliv Target Ther Agents. 2006;13:175–87. https://doi.org/10.1080/10717540500455975.
Sinico C, Fadda AM. Vesicular carriers for dermal drug delivery. Expert Opin Drug Deliv. 2009;6:813–25. https://doi.org/10.1517/17425240903071029.
Neubert RHH. Potentials of new nanocarriers for dermal and transdermal drug delivery. Eur J Pharm Biopharm. 2011;77:1–2. https://doi.org/10.1016/j.ejpb.2010.11.003.
Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev. 2012;64:175–93. https://doi.org/10.1016/j.addr.2012.09.018.
Bubic Pajic N, Nikolic I, Mitsou E, Papadimitriou V, Xenakis A, Randjelovic D, et al. Biocompatible microemulsions for improved dermal delivery of sertaconazole nitrate: phase behavior study and microstructure influence on drug biopharamaceutical properties. J Mol Liq. 2018;272:746–58. https://doi.org/10.1016/j.molliq.2018.10.002.
Poh Y, Ng S, Ho K. Formulation and characterisation of 1-ethyl-3-methylimidazolium acetate-in-oil microemulsions as the potential vehicle for drug delivery across the skin barrier. J Mol Liq. 2019;273:339–45. https://doi.org/10.1016/j.molliq.2018.10.034.
Froelich A, Osmałek T, Jadach B, Puri V, Michniak-Kohn B. Microemulsion-based media in nose-to-brain drug delivery. Pharmaceutics. 2021;13:1–37. https://doi.org/10.3390/pharmaceutics13020201.
Starýchová L, Žabka M, Špaglová M, Čuchorová M, Vitková M, Čierna M, et al. In vitro liberation of indomethacin from chitosan gels containing microemulsion in different dissolution mediums. J Pharm Sci. 2014;103:3977–84. https://doi.org/10.1002/jps.24213.
Shukla T, Upmanyu N, Agrawal M, Saraf S, Saraf S, Alexander A. Biomedical applications of microemulsion through dermal and transdermal route. Biomed Pharmacother. 2018;108:1477–94. https://doi.org/10.1016/j.biopha.2018.10.021.
Froelich A, Osmałek T, Snela A, Kunstman P, Jadach B, Olejniczak M, et al. Novel microemulsion-based gels for topical delivery of indomethacin: formulation, physicochemical properties and in vitro drug release studies. J Colloid Interface Sci. 2017;507:323–36. https://doi.org/10.1016/j.jcis.2017.08.011.
Zhang H, Zhao Z, Chen W, Lv M, Cheng J, Sun Z. In vitro and in vivo studies of micro-depots using tailored microemulsion for sustained local anaesthesia. Pharm Dev Technol. 2020;25:874–81. https://doi.org/10.1080/10837450.2020.1754425.
Üstündağ Okur N, Çağlar EŞ, Arpa MD, Karasulu HY. Preparation and evaluation of novel microemulsion-based hydrogels for dermal delivery of benzocaine. Pharm Dev Technol. 2017;22. https://doi.org/10.3109/10837450.2015.1131716.
Lamoudi L, Akretche S, Hadjsadok A, Daoud K. Fusidic acid microemulsion based on a pseudoternary phase diagram: development, characterization, and evaluation. J Pharm Innov. 2022. https://doi.org/10.1007/s12247-022-09668-4.
Üstündag-Okur N, Gökçe EH, Eǧrilmez S, Özer Ö, Ertan G. Novel ofloxacin-loaded microemulsion formulations for ocular delivery. J Ocul Pharmacol Ther. 2014;30:319–32. https://doi.org/10.1089/jop.2013.0114.
Moghimipour E, Salimi A, Eftekhari S. Design and characterization of microemulsion systems for naproxen. Adv Pharm Bull. 2013;3:63–71. https://doi.org/10.5681/apb.2013.011.
Elfiyani R, Amalia A, Pratama SY. Effect of using the combination of tween 80 and ethanol on the forming and physical stability of microemulsion of eucalyptus oil as antibacterial. J Young Pharm. 2017;9:S1-4. https://doi.org/10.5530/jyp.2017.1s.1.
ICH. ICH Harmonised Tripartite Guideline (2005) Validation of analytical procedures: text and methodology Q2(R1). International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Geneva, 1-13. 2005.
Şahin D, Çağlar EŞ, Boran T, Karadağ AE, Özhan G, Üstündağ Okur N. Development, characterization of naringenin-loaded promising microemulsion formulations, and demonstration of anti-aging efficacy by in vitro enzyme activity and gene expression. J Drug Deliv Sci Technol. 2023;84. https://doi.org/10.1016/j.jddst.2023.104422.
Çiftçi H, Arpa MD, Gülaçar İM, Özcan L, Ersoy B. Development and evaluation of mesoporous montmorillonite/magnetite nanocomposites loaded with 5-Fluorouracil. Microporous Mesoporous Mater. 2020;303. https://doi.org/10.1016/j.micromeso.2020.110253.
Ay Şenyiğit Z, Coşkunmeriç N, Çağlar EŞ, Öztürk İ, Atlıhan Gündoğdu E, Siafaka PI, et al. Chitosan-bovine serum albumin-Carbopol 940 nanogels for mupirocin dermal delivery: ex-vivo permeation and evaluation of cellular binding capacity via radiolabeling. Pharm Dev Technol. 2021;26:852–66. https://doi.org/10.1080/10837450.2021.1948570.
Okur ME, Karadağ AE, Okur NÜ, Özhan Y, Sipahi H, Ayla Ş, et al. In vivo wound healing and in vitro anti-inflammatory activity evaluation of phlomis russeliana extract gel formulations. Molecules. 2020;25:1–17. https://doi.org/10.3390/molecules25112695.
Okur ME, Karadağ AE, Özhan Y, Sipahi H, Ayla Ş, Daylan B, et al. Anti-inflammatory, analgesic and in vivo-in vitro wound healing potential of the Phlomis rigida Labill extract. J Ethnopharmacol Ireland. 2021;266:113408. https://doi.org/10.1016/j.jep.2020.113408.
Weinstein RD, Muske KR, Moriarty J, Schmidt EK. The solubility of benzocaine, lidocaine, and procaine in liquid and supercritical carbon dioxide. J Chem Eng Data. 2004;49:547–52. https://doi.org/10.1021/je034163p.
Nastiti CMRR, Ponto T, Abd E, Grice JE, Benson HAE, Roberts MS. Topical nano and microemulsions for skin delivery. Pharmaceutics. 2017;9. https://doi.org/10.3390/pharmaceutics9040037.
Xing Q, Song J, You X, Xu D, Wang K, Song J, et al. Microemulsions containing long-chain oil ethyl oleate improve the oral bioavailability of piroxicam by increasing drug solubility and lymphatic transportation simultaneously. Int J Pharm. 2016;511:709–18. https://doi.org/10.1016/j.ijpharm.2016.07.061.
Zhang YT, Zhao JH, Zhang SJ, Zhong YZ, Wang Z, Liu Y, et al. Enhanced transdermal delivery of evodiamine and rutaecarpine using microemulsion. Int J Nanomedicine. 2011;6:2469–82. https://doi.org/10.2147/ijn.s25258.
Tang H, Xiang S, Li X, Zhou J, Kuang C. Preparation and in vitro performance evaluation of resveratrol for oral self-microemulsion. PLoS ONE. 2019;14:1–17. https://doi.org/10.1371/journal.pone.0214544.
Turnidge J. Fusidic acid pharmacology, pharmacokinetics and pharmacodynamics. Int J Antimicrob Agents. 1999;12. https://doi.org/10.1016/S0924-8579(98)00071-5.
Barham WT, Katherine M. The effect of the fish anaesthetic benzocaine hydrochloride on the quality of saline water. Water SA. 1980;6:207.
Lambers H, Piessens S, Bloem A, Pronk H, Finkel P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int J Cosmet Sci. 2006;28:359–70. https://doi.org/10.1111/j.1467-2494.2006.00344.x.
Pan Q, Fan R, Chen R, Yuan J, Chen S, Cheng B. Weakly acidic microenvironment of the wound bed boosting the efficacy of acidic fibroblast growth factor to promote skin regeneration. Front Bioeng Biotechnol. 2023;11:1–11. https://doi.org/10.3389/fbioe.2023.1150819.
Badawi AA, Nour SA, Sakran WS, El-Mancy SMS. Preparation and evaluation of microemulsion systems containing salicylic acid. AAPS PharmSciTech. 2009;10:1081–4. https://doi.org/10.1208/s12249-009-9301-7.
Yozgatlı V, Üstündağ Okur N, Okur ME, Sipahi H, Charehsaz M, Aydın A, et al. Managing allergic conjunctivitis via ophthalmic microemulsions: formulation, characterization, in vitro irritation studies based on EpiOcularTM eye irritation assay and in vivo studies in rabbit eye. J Surfactants Deterg. 2023;1–14. https://doi.org/10.1002/jsde.12691.
Ciríaco SL, Carvalho IPS, Alves Terceiro Neto J, de Sousa Lima Neto J, de Oliveira DHB, Cunha APGP, et al. Development of microemulsion of tamsulosin and dutasteride for benign prostatic hyperplasia therapy. Colloids Surfaces B Biointerfaces. 2020;185:110573. https://doi.org/10.1016/j.colsurfb.2019.110573.
Çağlar EŞ, Okur ME, Aksu B, Üstündağ ON. Transdermal delivery of acemetacin loaded microemulsions: preparation, characterization, in vitro–ex vivo evaluation and in vivo analgesic and anti-inflammatory efficacy. J Dispers Sci Technol. 2023. https://doi.org/10.1080/01932691.2023.2175691.
Kadu PJ, Kushare SS, Thacker DD, Gattani SG. Enhancement of oral bioavailability of atorvastatin calcium by self-emulsifying drug delivery systems (SEDDS). Pharm Dev Technol. 2011;16:65–74. https://doi.org/10.3109/10837450903499333.
Subongkot T, Ngawhirunpat T. Development of a novel microemulsion for oral absorption enhancement of all-trans retinoic acid. Int J Nanomedicine. 2017;12:5585–99. https://doi.org/10.2147/IJN.S142503.
Sabale V, Vora S. Formulation and evaluation of microemulsion-based hydrogel for topical delivery. Int J Pharm Investig. 2012;2:140. https://doi.org/10.4103/2230-973x.104397.
Patel V, Kukadiya H, Mashru R, Surti N, Mandal S. Development of microemulsion for solubility enhancement of clopidogrel. Iran J Pharm Res. 2010;9:327–34.
Manyala DL, Ghosh M, Dalai S. Novel microemulsions formulated from sodium N-Lauroyl sarcosinate as nanocarrier for encapsulation of α-Tocopherol. J Mol Liq. 2023;384: 122323. https://doi.org/10.1016/j.molliq.2023.122323.
Goindi S, Narula M, Kalra A. Microemulsion-based topical hydrogels of tenoxicam for treatment of arthritis. AAPS PharmSciTech. 2016;17:597–606. https://doi.org/10.1208/s12249-015-0383-0.
Monton C, Settharaksa S, Suksaeree J, Chusut T. The preparation, characterization, and stability evaluation of a microemulsion-based oral spray containing clove oil for the treatment of oral candidiasis. J Drug Deliv Sci Technol. 2020;57. https://doi.org/10.1016/j.jddst.2020.101735.
Karasulu HY. Microemulsions as novel drug carriers: the formation, stability, applications and toxicity. Expert Opin Drug Deliv. 2008;5:119–35. https://doi.org/10.1517/17425247.5.1.119.
Karasulu HY, Oruç N, Üstündağ Okur N, Ilem Özdemir D, Ay Şenyiğit Z, Barbet Yilmaz F, et al. Aprotinin revisited: formulation, characterization, biodistribution and therapeutic potential of new aprotinin microemulsion in acute pancreatitis. J Drug Target. 2015;23:525–37. https://doi.org/10.3109/1061186X.2015.1015537.
Üstündağ Okur N, Yavasoglu A, Karasulu HY. Preparation and evaluation of microemulsion formulations of naproxen for dermal delivery. Chem Pharm Bull. 2014;62:135–43. https://doi.org/10.1248/cpb.c13-00051.
Callender SP, Mathews JA, Kobernyk K, Wettig SD. Microemulsion utility in pharmaceuticals: ımplications for multi-drug delivery. Int J Pharm. 2017;526:425–42. https://doi.org/10.1016/j.ijpharm.2017.05.005.
Suhaimi SH, Hasham R, Rosli NA. Effects of formulation parameters on particle size and polydispersity ındex of orthosiphon stamineus loaded nanostructured lipid carrier. J Adv Res Appl Sci Eng Technol ISSN. 2015;1:36–9.
Takechi-Haraya Y, Ohgita T, Demizu Y, Saito H, Izutsu K ichi, Sakai-Kato K. Current status and challenges of analytical methods for evaluation of size and surface modification of nanoparticle-based drug formulations. AAPS PharmSciTech. 2022;23. https://doi.org/10.1208/s12249-022-02303-y.
Kogan A, Garti N. Microemulsions as transdermal drug delivery vehicles. Adv Colloid Interface Sci. 2006;123–126:369–85. https://doi.org/10.1016/j.cis.2006.05.014.
Lopes LB. Overcoming the cutaneous barrier with microemulsions. Pharmaceutics. 2014;6:52–77. https://doi.org/10.3390/pharmaceutics6010052.
Malacarne MC, Banfi S, Rugiero M, Caruso E. Drug delivery systems for the photodynamic application of two photosensitizers belonging to the porphyrin family. Photochem Photobiol Sci. 2021;20:1011–25. https://doi.org/10.1007/s43630-021-00076-0.
Rowe RC, Sheskey PJ, Owen SC. Handbook of pharmaceutical excipients. 6th ed. London: Washington, DC: Pharmaceutical Press; 2009.
Arpa MD, Ünükür MZ, Erim ÜC. Formulation, characterization and in vitro release studies of terbinafine hydrochloride loaded buccal films. J Res Pharm. 2021;25:667–80. https://doi.org/10.29228/jrp.58.
Alireza Mortazavi S, Pishrochi S, Jafari Azar Z. Formulation and in-vitro evaluation of tretinoin microemulsion as a potential carrier for dermal drug delivery. Iran J Pharm Res. 2013;12:599–609. https://doi.org/10.22037/ijpr.2013.1391.
Lalan MS, Laddha NC, Lalani J, Imran MJ, Begum R, Misra A. Dose reduction of a potent topical corticosteroid with microemulsion based cream. J Nanopharmaceut Drug Deliv. 2013;1:52–63. https://doi.org/10.1166/jnd.2013.1008.
Üstündağ Okur N, Çağlar EŞ, Okur ME, Ayla Ş. Evaluation of hydroquinone loaded microemulsion formulations for melasma treatment. 2019;23:662–70. https://doi.org/10.12991/jrp.2019.174
Rao S, Barot T, Rajesh KS, Jha LL. Formulation, optimization and evaluation of microemulsion based gel of butenafine hydrochloride for topical delivery by using simplex lattice mixture design. J Pharm Investig. 2016;46:1–12. https://doi.org/10.1007/s40005-015-0207-y.
Arpa MD, Seçen İM, Erim ÜC, Hoş A, Üstündağ ON. Azelaic acid loaded chitosan and HPMC based hydrogels for treatment of acne: formulation, characterization, in vitro-ex vivo evaluation. Pharm Dev Technol. 2022;27:268–81. https://doi.org/10.1080/10837450.2022.2038620.
Coneac G, Vlaia V, Olariu I, Muţ AM, Anghel DF, Ilie C, et al. Development and evaluation of new microemulsion-based hydrogel formulations for topical delivery of fluconazole. AAPS PharmSciTech. 2015;16:889–904. https://doi.org/10.1208/s12249-014-0275-8.
Malakar J, Nayak AK, Basu A. Ondansetron HCl Microemulsions for transdermal delivery: formulation and ın vitro skin permeation. ISRN Pharm. 2012;2012:1–6. https://doi.org/10.5402/2012/428396.
Camelo SRP, Franceschi S, Perez E, Fullana SG, Ré MI. Factors influencing the erosion rate and the drug release kinetics from organogels designed as matrices for oral controlled release of a hydrophobic drug. Drug Dev Ind Pharm. 2016;42:985–97. https://doi.org/10.3109/03639045.2015.1103746.
Laxmi M, Bhardwaj A, Mehta S, Mehta A. Development and characterization of nanoemulsion as carrier for the enhancement of bioavailability of artemether. Artif Cells, Nanomedicine Biotechnol. 2015;43:334–44. https://doi.org/10.3109/21691401.2014.887018.
Lipsky BA, Hoey C. Topical antimicrobial therapy for treating chronic wounds. Clin Infect Dis. 2009;49:1541–9. https://doi.org/10.1086/644732.
Chhibber T, Wadhwa S, Chadha P, Sharma G, Katare OP. Phospholipid structured microemulsion as effective carrier system with potential in methicillin sensitive Staphylococcus aureus (MSSA) involved burn wound infection. J Drug Target. 2015;23:943–52. https://doi.org/10.3109/1061186X.2015.1048518.
Chhibber S, Gondil VS, Singla L, Kumar M, Chhibber T, Sharma G, et al. Effective topical delivery of H-AgNPs for eradication of Klebsiella pneumoniae–ınduced burn wound ınfection. AAPS PharmSciTech. 2019;20:1–13. https://doi.org/10.1208/s12249-019-1350-y.
Almostafa MM, Elsewedy HS, Shehata TM, Soliman WE. Novel formulation of fusidic acid ıncorporated into a myrrh-oil-based nanoemulgel for the enhancement of skin bacterial ınfection treatment. Gels. 2022;8. https://doi.org/10.3390/gels8040245.
Bolla SR, Mohammed Al-Subaie A, Yousuf Al-Jindan R, Papayya Balakrishna J, Kanchi Ravi P, Veeraraghavan VP, et al. In vitro wound healing potency of methanolic leaf extract of Aristolochia saccata is possibly mediated by its stimulatory effect on collagen-1 expression. Heliyon. 2019;5: e01648. https://doi.org/10.1016/j.heliyon.2019.e01648.
Nosrati H, Heydari M, Tootiaei Z, Ganjbar S, Khodaei M. Delivery of antibacterial agents for wound healing applications using polysaccharide-based scaffolds. J Drug Deliv Sci Technol. 2023;84: 104516. https://doi.org/10.1016/j.jddst.2023.104516.
Pitz HDS, Pereira A, Blasius MB, Voytena APL, Affonso RCL, Fanan S, et al. In vitro evaluation of the antioxidant activity and wound healing properties of Jaboticaba (Plinia peruviana) fruit peel hydroalcoholic extract. Oxid Med Cell Longev. 2016. https://doi.org/10.1155/2016/3403586.
Reis R, Sipahi H, Dinc O, Kavaz T, Charehsaz M, Dimoglo A, et al. Toxicity, mutagenicity and stability assessment of simply produced electrolyzed water as a wound healing agent in vitro. Hum Exp Toxicol. 2021;40:452–63. https://doi.org/10.1177/0960327120952151.
Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–33. https://doi.org/10.1038/nprot.2007.30.
Acknowledgements
The authors thank Berko İlaç (Türkiye) for donating fusidic acid.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
Muhammet Davut Arpa: investigation, methodology, funding acquisition, writing—original draft, writing–review and editing; Emre Şefik Çağlar: investigation, methodology, funding acquisition, writing—original draft; Dilara Güreşçi: investigation, methodology, writing—original draft; Hande Sipahi: investigation, methodology, funding acquisition, writing—original draft; Neslihan Üstündağ Okur: supervision, project administration, investigation, methodology, funding acquisition, writing—original draft, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arpa, M.D., Çağlar, E.Ş., Güreşçi, D. et al. Novel Microemulsion Containing Benzocaine and Fusidic Acid Simultaneously: Formulation, Characterization, and In Vitro Evaluation for Wound Healing. AAPS PharmSciTech 25, 53 (2024). https://doi.org/10.1208/s12249-024-02762-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-024-02762-5