Abstract
Formononetin is a flavonoid compound with anti-tumor and anti-inflammatory properties. However, its low solubility limits its clinical use. We employed microfluidic technology to prepare formononetin-loaded PLGA-PEGDA microspheres (Degradable polymer PLGA, Crosslinking agent PEGDA), which can encapsulate and release drugs in a controlled manner. We optimized and characterized the microspheres, and evaluated their antitumor effects. The microspheres had uniform size, high drug loading efficiency, high encapsulation efficiency, and stable release for 35 days. They also inhibited the proliferation, migration, and apoptosis. The antitumor mechanism involved the induction of reactive oxygen species and modulation of Bcl-2 family proteins. These findings suggested that formononetin-loaded PLGA-PEGDA microspheres, created using microfluidic technology, could be a novel drug delivery system that can overcome the limitations of formononetin and enhance its antitumor activity.
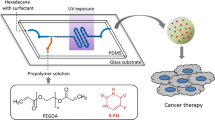
Graphical Abstract









Similar content being viewed by others
References
Zhang S, Sun J. Nano-drug delivery system for the treatment of acute myelogenous leukemia. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2022;51(2):233–40. https://doi.org/10.3724/zdxbyxb-2022-0084.
Qamar Z, Qizilbash FF, Iqubal MK, Ali A, Narang JK, Ali J, et al. Nano-based drug delivery system: recent strategies for the treatment of ocular disease and future perspective. Recent Pat Drug Deliv Formul. 2019;13(4):246–54. https://doi.org/10.2174/1872211314666191224115211.
Li B, Shao H, Gao L, Li H, Sheng H, Zhu L. Nano-drug co-delivery system of natural active ingredients and chemotherapy drugs for cancer treatment: a review. Drug Deliv. 2022;29(1):2130–61. https://doi.org/10.1080/10717544.2022.2094498.
Xie M, Xu Y, Shen H, Shen S, Ge Y, Xie J. Negative-charge-functionalized mesoporous silica nanoparticles as drug vehicles targeting hepatocellular carcinoma. Int J Pharm. 2014;474(1-2):223–31. https://doi.org/10.1016/j.ijpharm.2014.08.027.
Rui M, Xing Y, Li R, Ge Y, Feng C, Xu X. Targeted biomimetic nanoparticles for synergistic combination chemotherapy of paclitaxel and doxorubicin. Molecular Pharmaceutics. 2017;14(1):107–23. https://doi.org/10.1021/acs.molpharmaceut.6b00732.
Hussein HA, Nazir MS, Azra N, Qamar Z, Seeni A, Tengku Din T, et al. Novel drug and gene delivery system and imaging agent based on marine diatom biosilica nanoparticles. Mar Drugs. 2022;20(8) https://doi.org/10.3390/md20080480.
Machado Dutra J, Espitia PJP, Andrade BR. Formononetin: biological effects and uses - a review. Food Chem. 2021;359:129975. https://doi.org/10.1016/j.foodchem.2021.129975.
Mendonça MAA, Ribeiro ARS, Lima AK, Bezerra GB, Pinheiro MS, Albuquerque-Júnior RLC, et al. Red propolis and its dyslipidemic regulator formononetin: evaluation of antioxidant activity and gastroprotective effects in rat model of gastric ulcer. Nutrients. 2020;12(10) https://doi.org/10.3390/nu12102951.
Zhang B, Hao Z, Zhou W, Zhang S, Sun M, Li H, et al. Formononetin protects against ox-LDL-induced endothelial dysfunction by activating PPAR-γ signaling based on network pharmacology and experimental validation. Bioengineered. 2021;12(1):4887–98. https://doi.org/10.1080/21655979.2021.1959493.
Tian J, Wang XQ, Tian Z. Focusing on formononetin: recent perspectives for its neuroprotective potentials. Front Pharmacol. 2022;13:905898. https://doi.org/10.3389/fphar.2022.905898.
Tay KC, Tan LT, Chan CK, Hong SL, Chan KG, Yap WH, et al. Formononetin: a review of its anticancer potentials and mechanisms. Front Pharmacol. 2019;10:820. https://doi.org/10.3389/fphar.2019.00820.
Yu L, Zhang Y, Chen Q, He Y, Zhou H, Wan H, et al. Formononetin protects against inflammation associated with cerebral ischemia-reperfusion injury in rats by targeting the JAK2/STAT3 signaling pathway. Biomed Pharmacother. 2022;149:112836. https://doi.org/10.1016/j.biopha.2022.112836.
Ma X, Wang J. Formononetin: a pathway to protect neurons. Front Integr Neurosci. 2022;16:908378. https://doi.org/10.3389/fnint.2022.908378.
Wang QY, Meng QH, Zhang ZT, Tian ZJ, Liu H. Synthesis, solubility, lipids-lowering and liver-protection activities of sulfonated formononetin. Yao Xue Xue Bao. 2009;44(4):386–9.
Ong SKL, Shanmugam MK, Fan L, Fraser SE, Arfuso F, Ahn KS, et al. Focus on formononetin: anticancer potential and molecular targets. Cancers (Basel). 2019;11(5) https://doi.org/10.3390/cancers11050611.
Zhang J, Liu L, Wang J, Ren B, Zhang L, Li W. Formononetin, an isoflavone from Astragalus membranaceus inhibits proliferation and metastasis of ovarian cancer cells. J Ethnopharmacol. 2018;221:91–9. https://doi.org/10.1016/j.jep.2018.04.014.
Almatroodi SA, Almatroudi A, Khan AA, Rahmani AH. Potential therapeutic targets of formononetin, a type of methoxylated isoflavone, and its role in cancer therapy through the modulation of signal transduction pathways. Int J Mol Sci. 2023;24(11) https://doi.org/10.3390/ijms24119719.
Yang Y, Zhao Y, Ai X, Cheng B, Lu S. Formononetin suppresses the proliferation of human non-small cell lung cancer through induction of cell cycle arrest and apoptosis. Int J Clin Exp Pathol. 2014;7(12):8453–61.
Zhang L, Gong Y, Wang S, Gao F. Anti-colorectal cancer mechanisms of formononetin identified by network pharmacological approach. Med Sci Monit. 2019;25:7709–14. https://doi.org/10.12659/msm.919935.
Li S, Zhu L, He Y, Sun T. Formononetin enhances the chemosensitivity of triple negative breast cancer via BTB domain and CNC homolog 1-mediated mitophagy pathways. Acta Biochim Pol. 2023;70(3):533–9. https://doi.org/10.18388/abp.2020_6466.
Wang Y, Deng Z, Wang X, Shi Y, Lu Y, Fang S, et al. Formononetin/methyl-β-cyclodextrin inclusion complex incorporated into electrospun polyvinyl-alcohol nanofibers: enhanced water solubility and oral fast-dissolving property. Int J Pharm. 2021;603:120696. https://doi.org/10.1016/j.ijpharm.2021.120696.
Kim JH, Kang DW, Cho SJ, Cho HY. Parent-metabolite pharmacokinetic modeling of formononetin and its active metabolites in rats after oral administration of formononetin formulations. Pharmaceutics. 2022;15(1) https://doi.org/10.3390/pharmaceutics15010045.
Zhang HY, Firempong CK, Wang YW, Xu WQ, Wang MM, Cao X, et al. Ergosterol-loaded poly(lactide-co-glycolide) nanoparticles with enhanced in vitro antitumor activity and oral bioavailability. Acta Pharmacologica Sinica. 2016;37(6):834–44. https://doi.org/10.1038/aps.2016.37.
Cao X, Deng W, Wei Y, Su W, Yang Y, Wei Y, et al. Encapsulation of plasmid DNA in calcium phosphate nanoparticles: stem cell uptake and gene transfer efficiency. Int J Nanomedicine. 2011;6:3335–49. https://doi.org/10.2147/ijn.S27370.
Bian J, Cai F, Chen H, Tang Z, Xi K, Tang J, et al. Modulation of local overactive inflammation via injectable hydrogel microspheres. Nano Lett. 2021;21(6):2690–8. https://doi.org/10.1021/acs.nanolett.0c04713.
Yang J, Han Y, Lin J, Zhu Y, Wang F, Deng L, et al. Ball-bearing-inspired polyampholyte-modified microspheres as bio-lubricants attenuate osteoarthritis. Small. 2020;16(44):e2004519. https://doi.org/10.1002/smll.202004519.
Zhang Q, Yang T, Zhang R, Liang X, Wang G, Tian Y, et al. Platelet lysate functionalized gelatin methacrylate microspheres for improving angiogenesis in endodontic regeneration. Acta Biomater. 2021;136:441–55. https://doi.org/10.1016/j.actbio.2021.09.024.
Wong CY, Al-Salami H, Dass CR. Microparticles, microcapsules and microspheres: a review of recent developments and prospects for oral delivery of insulin. Int J Pharm. 2018;537(1-2):223–44. https://doi.org/10.1016/j.ijpharm.2017.12.036.
Li W, Chen J, Zhao S, Huang T, Ying H, Trujillo C, et al. High drug-loaded microspheres enabled by controlled in-droplet precipitation promote functional recovery after spinal cord injury. Nat Commun. 2022;13(1):1262. https://doi.org/10.1038/s41467-022-28787-7.
Zhang Y, Shen L, Wang T, Li H, Huang R, Zhang Z, et al. Taste masking of water-soluble drug by solid lipid microspheres: a child-friendly system established by reversed lipid-based nanoparticle technique. J Pharm Pharmacol. 2020;72(6):776–86. https://doi.org/10.1111/jphp.13245.
Gouerou H, Dain MP, Parrondo I, Poisson D, Bernades P. Alipase versus nonenteric-coated enzymes in pancreatic insufficiency. A French multicenter crossover comparative study. Int J Pancreatol. 1989;5(Suppl):45–50.
Su Y, Liu J, Tan S, Liu W, Wang R, Chen C. PLGA sustained-release microspheres loaded with an insoluble small-molecule drug: microfluidic-based preparation, optimization, characterization, and evaluation in vitro and in vivo. Drug Deliv. 2022;29(1):1437–46. https://doi.org/10.1080/10717544.2022.2072413.
Ghosh Dastidar D, Saha S, Chowdhury M. Porous microspheres: synthesis, characterisation and applications in pharmaceutical & medical fields. Int J Pharm. 2018;548(1):34–48. https://doi.org/10.1016/j.ijpharm.2018.06.015.
Larsen LI, López GP, Selwyn R, Carroll NJ. Microfluidic fabrication of silica microspheres infused with positron emission tomography imaging agents. ACS Appl Bio Mater. 2023;6(2):712–21. https://doi.org/10.1021/acsabm.2c00940.
Cao X, Liu Q, Adu-Frimpong M, Shi W, Liu K, Deng T, et al. Microfluidic generation of near-infrared photothermal vitexin/ICG liposome with amplified photodynamic therapy. AAPS PharmSciTech. 2023;24(4):82. https://doi.org/10.1208/s12249-023-02539-2.
Zhao Q, Cui H, Wang Y, Du X. Microfluidic platforms toward rational material fabrication for biomedical applications. Small. 2020;16(9):e1903798. https://doi.org/10.1002/smll.201903798.
Lin Z, Rao Z, Chen J, Chu H, Zhou J, Yang L, et al. Bioactive decellularized extracellular matrix hydrogel microspheres fabricated using a temperature-controlling microfluidic system. ACS Biomater Sci Eng. 2022;8(4):1644–55. https://doi.org/10.1021/acsbiomaterials.1c01474.
Amini H, Lee W, Di Carlo D. Inertial microfluidic physics. Lab Chip. 2014;14(15):2739–61. https://doi.org/10.1039/c4lc00128a.
Yao Y, Lin JJ, Chee XYJ, Liu MH, Khan SA, Kim JE. Encapsulation of lutein via microfluidic technology: evaluation of stability and in vitro bioaccessibility. Foods. 2021;10(11) https://doi.org/10.3390/foods10112646.
Seeto WJ, Tian Y, Pradhan S, Kerscher P, Lipke EA. Rapid production of cell-laden microspheres using a flexible microfluidic encapsulation platform. Small. 2019;15(47):e1902058. https://doi.org/10.1002/smll.201902058.
Bolze H, Erfle P, Riewe J, Bunjes H, Dietzel A, Burg TP. A microfluidic split-flow technology for product characterization in continuous low-volume nanoparticle synthesis. Micromachines (Basel). 2019;10(3) https://doi.org/10.3390/mi10030179.
Wu S, Wang Z, Wang Y, Guo M, Zhou M, Wang L, et al. Peptide-grafted microspheres for mesenchymal stem cell sorting and expansion by selective adhesion. Front Bioeng Biotechnol. 2022;10:873125. https://doi.org/10.3389/fbioe.2022.873125.
Zhou W, Dou M, Timilsina SS, Xu F, Li X. Recent innovations in cost-effective polymer and paper hybrid microfluidic devices. Lab Chip. 2021;21(14):2658–83. https://doi.org/10.1039/d1lc00414j.
Siavashy S, Soltani M, Ghorbani-Bidkorbeh F, Fallah N, Farnam G, Mortazavi SA, et al. Microfluidic platform for synthesis and optimization of chitosan-coated magnetic nanoparticles in cisplatin delivery. Carbohydr Polym. 2021;265:118027. https://doi.org/10.1016/j.carbpol.2021.118027.
Hernández-Giottonini KY, Rodríguez-Córdova RJ, Gutiérrez-Valenzuela CA, Peñuñuri-Miranda O, Zavala-Rivera P, Guerrero-Germán P, et al. PLGA nanoparticle preparations by emulsification and nanoprecipitation techniques: effects of formulation parameters. RSC Adv. 2020;10(8):4218–31. https://doi.org/10.1039/c9ra10857b.
Wang S, Liang WF, Dong ZL, Lee VGB, Li WJ. Fabrication of micrometer- and nanometer-scale polymer structures by visible light induced dielectrophoresis (DEP) force. Micromachines. 2011;2(4):431–42. https://doi.org/10.3390/mi2040431.
Blasi P. Poly(lactic acid)/poly(lactic-co-glycolic acid)-based microparticles: an overview. Journal of Pharmaceutical Investigation. 2019;49(4):337–46. https://doi.org/10.1007/s40005-019-00453-z.
He C, Zeng W, Su Y, Sun R, Xiao Y, Zhang B, et al. Microfluidic-based fabrication and characterization of drug-loaded PLGA magnetic microspheres with tunable shell thickness. Drug Deliv. 2021;28(1):692–9. https://doi.org/10.1080/10717544.2021.1905739.
Chen M, Aluunmani R, Bolognesi G, Vladisavljević GT. Facile microfluidic fabrication of biocompatible hydrogel microspheres in a novel microfluidic device. Molecules. 2022:27(13). https://doi.org/10.3390/molecules27134013.
Wang X, Wang L, Qi F, Zhao J. The effect of a single injection of uniform-sized insulin-loaded PLGA microspheres on peri-implant bone formation. RSC Adv. 2018;8(70):40417–25. https://doi.org/10.1039/c8ra08505f.
Li X, Xia X, Zhang J, Adu-Frimpong M, Shen X, Yin W, et al. Preparation, physical characterization, pharmacokinetics and anti-hyperglycemic activity of esculetin-loaded mixed micelles. J Pharm Sci. 2023;112(1):148–57. https://doi.org/10.1016/j.xphs.2022.06.022.
Zhu Z, Liu J, Yang Y, Adu-Frimpong M, Ji H, Toreniyazov E, et al. SMEDDS for improved oral bioavailability and anti-hyperuricemic activity of licochalcone A. J Microencapsul. 2021;38(7-8):459–71. https://doi.org/10.1080/02652048.2021.1963341.
Jafarifar E, Hajialyani M, Akbari M, Rahimi M, Shokoohinia Y, Fattahi A. Preparation of a reproducible long-acting formulation of risperidone-loaded PLGA microspheres using microfluidic method. Pharm Dev Technol. 2017;22(6):836–43. https://doi.org/10.1080/10837450.2016.1221426.
El-Didamony SE, Amer RI, El-Osaily GH. Formulation, characterization and cellular toxicity assessment of a novel bee-venom microsphere in prostate cancer treatment. Sci Rep. 2022;12(1):13213. https://doi.org/10.1038/s41598-022-17391-w.
Cao X, Zhu Q, Wang QL, Adu-Frimpong M, Wei CM, Weng W, et al. Improvement of oral bioavailability and anti-tumor effect of zingerone self-microemulsion drug delivery system. J Pharm Sci. 2021;110(7):2718–27. https://doi.org/10.1016/j.xphs.2021.01.037.
Yi C, Zhong H, Tong S, Cao X, Firempong CK, Liu H, et al. Enhanced oral bioavailability of a sterol-loaded microemulsion formulation of Flammulina velutipes, a potential antitumor drug. International Journal of Nanomedicine. 2012;7:5067–78. https://doi.org/10.2147/ijn.S34612.
Marulanda K, Brokaw D, Gambarian M, Pareta R, McQuilling JP, Opara EC, et al. Controlled delivery of Slit3 proteins from alginate microbeads inhibits in vitro angiogenesis. J Surg Res. 2021;264:90–8. https://doi.org/10.1016/j.jss.2021.01.025.
Pijuan J, Barceló C, Moreno DF, Maiques O, Sisó P, Marti RM, et al. In vitro cell migration, invasion, and adhesion assays: from cell imaging to data analysis. Front Cell Dev Biol. 2019;7:107. https://doi.org/10.3389/fcell.2019.00107.
Ayyanaar S, Kesavan MP, Balachandran C, Rasala S, Rameshkumar P, Aoki S, et al. Iron oxide nanoparticle core-shell magnetic microspheres: applications toward targeted drug delivery. Nanomedicine. 2020;24:102134. https://doi.org/10.1016/j.nano.2019.102134.
Sun C, Li W, Liu Y, Deng W, Adu-Frimpong M, Zhang H, et al. In vitro/in vivo hepatoprotective properties of 1-O-(4-hydroxymethylphenyl)-α-L-rhamnopyranoside from Moringa oleifera seeds against carbon tetrachloride-induced hepatic injury. Food Chem Toxicol. 2019;131:110531. https://doi.org/10.1016/j.fct.2019.05.039.
Hu W, Xiao Z. Formononetin induces apoptosis of human osteosarcoma cell line U2OS by regulating the expression of Bcl-2, Bax and MiR-375 in vitro and in vivo. Cell Physiol Biochem. 2015;37(3):933–9. https://doi.org/10.1159/000430220.
Aladaileh SH, Hussein OE, Abukhalil MH, Saghir SAM, Bin-Jumah M, Alfwuaires MA, et al. Formononetin upregulates Nrf2/HO-1 signaling and prevents oxidative stress, inflammation, and kidney injury in methotrexate-induced rats. Antioxidants (Basel). 2019;8(10) https://doi.org/10.3390/antiox8100430.
Sun X, Lv W, Wang Y, Zhang X, Ouyang Z, Yin R, et al. Mrgprb2 gene plays a role in the anaphylactoid reactions induced by Houttuynia cordata injection. Journal of Ethnopharmacology. 2022:289. https://doi.org/10.1016/j.jep.2022.115053.
Shi W, Cao X, Liu Q, Zhu Q, Liu K, Deng T, et al. Hybrid membrane-derived nanoparticles for isoliquiritin enhanced glioma therapy. Pharmaceuticals (Basel). 2022;15(9) https://doi.org/10.3390/ph15091059.
Cao X, Deng T, Zhu Q, Wang J, Shi W, Liu Q, et al. Photothermal therapy mediated hybrid membrane derived nano-formulation for enhanced cancer therapy. AAPS PharmSciTech. 2023;24(6):146. https://doi.org/10.1208/s12249-023-02594-9.
Ding D, Zhu Q. Recent advances of PLGA micro/nanoparticles for the delivery of biomacromolecular therapeutics. Mater Sci Eng C Mater Biol Appl. 2018;92:1041–60. https://doi.org/10.1016/j.msec.2017.12.036.
Mahar R, Chakraborty A, Nainwal N, Bahuguna R, Sajwan M, Jakhmola V. Application of PLGA as a biodegradable and biocompatible polymer for pulmonary delivery of drugs. AAPS PharmSciTech. 2023;24(1):39. https://doi.org/10.1208/s12249-023-02502-1.
Hajavi J, Ebrahimian M, Sankian M, Khakzad MR, Hashemi M. Optimization of PLGA formulation containing protein or peptide-based antigen: recent advances. J Biomed Mater Res A. 2018;106(9):2540–51. https://doi.org/10.1002/jbm.a.36423.
Abdul Rahim R, Jayusman PA, Muhammad N, Ahmad F, Mokhtar N, Naina Mohamed I, et al. Recent advances in nanoencapsulation systems using PLGA of bioactive phenolics for protection against chronic diseases. Int J Environ Res Public Health. 2019;16(24) https://doi.org/10.3390/ijerph16244962.
Butreddy A, Gaddam RP, Kommineni N, Dudhipala N, Voshavar C. PLGA/PLA-based long-acting injectable depot microspheres in clinical use: production and characterization overview for protein/peptide delivery. Int J Mol Sci. 2021;22(16) https://doi.org/10.3390/ijms22168884.
Muddineti OS, Omri A. Current trends in PLGA based long-acting injectable products: the industry perspective. Expert Opin Drug Deliv. 2022;19(5):559–76. https://doi.org/10.1080/17425247.2022.2075845.
McAvoy K, Jones D, Thakur RRS. Synthesis and characterisation of photocrosslinked poly(ethylene glycol) diacrylate implants for sustained ocular drug delivery. Pharm Res. 2018;35(2):36. https://doi.org/10.1007/s11095-017-2298-9.
Sabel-Grau T, Tyushina A, Babalik C, Lensen MC. UV-VIS curable PEG hydrogels for biomedical applications with multifunctionality. Gels. 2022;8(3) https://doi.org/10.3390/gels8030164.
Bhardwaj VK, Purohit R. A comparative study on inclusion complex formation between formononetin and β-cyclodextrin derivatives through multiscale classical and umbrella sampling simulations. Carbohydr Polym. 2023;310:120729. https://doi.org/10.1016/j.carbpol.2023.120729.
Obaidat R, BaniAmer F, Assaf SM, Yassin A. Fabrication and evaluation of transdermal delivery of carbamazepine dissolving microneedles. AAPS PharmSciTech. 2021;22(8):253. https://doi.org/10.1208/s12249-021-02136-1.
Liu J, Wang Q, Omari-Siaw E, Adu-Frimpong M, Liu J, Xu X, et al. Enhanced oral bioavailability of bisdemethoxycurcumin-loaded self-microemulsifying drug delivery system: formulation design, in vitro and in vivo evaluation. Int J Pharm. 2020;590:119887. https://doi.org/10.1016/j.ijpharm.2020.119887.
Wang Q, Wei Q, Yang Q, Cao X, Li Q, Shi F, et al. A novel formulation of [6]-gingerol: proliposomes with enhanced oral bioavailability and antitumor effect. Int J Pharm. 2018;535(1-2):308–15. https://doi.org/10.1016/j.ijpharm.2017.11.006.
Löf D, Schillén K, Nilsson L. Flavonoids: precipitation kinetics and interaction with surfactant micelles. J Food Sci. 2011;76(3):N35–9. https://doi.org/10.1111/j.1750-3841.2011.02103.x.
Kim MS, Park JS, Chung YC, Jang S, Hyun CG, Kim SY. Anti-inflammatory effects of formononetin 7-O-phosphate, a novel biorenovation product, on LPS-stimulated RAW 264.7 macrophage cells. Molecules. 2019;24(21) https://doi.org/10.3390/molecules24213910.
Karmakar J, Mukherjee K, Mandal C. Siglecs modulate activities of immune cells through positive and negative regulation of ROS generation. Front Immunol. 2021;12:758588. https://doi.org/10.3389/fimmu.2021.758588.
He L, He T, Farrar S, Ji L, Liu T, Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol Biochem. 2017;44(2):532–53. https://doi.org/10.1159/000485089.
Li T, Gao SJ. KSHV hijacks FoxO1 to promote cell proliferation and cellular transformation by antagonizing oxidative stress. J Med Virol. 2023;95(3):e28676. https://doi.org/10.1002/jmv.28676.
Rahman MA, Ahmed KR, Haque F, Park MN, Kim B. Recent advances in cellular signaling interplay between redox metabolism and autophagy modulation in cancer: an overview of molecular mechanisms and therapeutic interventions. Antioxidants (Basel). 2023;12(2) https://doi.org/10.3390/antiox12020428.
Zhan Y, Zhang Z, Liu Y, Fang Y, Xie Y, Zheng Y, et al. NUPR1 contributes to radiation resistance by maintaining ROS homeostasis via AhR/CYP signal axis in hepatocellular carcinoma. BMC Med. 2022;20(1):365. https://doi.org/10.1186/s12916-022-02554-3.
Sun C, Li W, Ma P, Li Y, Zhu Y, Zhang H, et al. Development of TPGS/F127/F68 mixed polymeric micelles: enhanced oral bioavailability and hepatoprotection of syringic acid against carbon tetrachloride-induced hepatotoxicity. Food Chem Toxicol. 2020;137:111126. https://doi.org/10.1016/j.fct.2020.111126.
Lai PK, Chan JY, Cheng L, Lau CP, Han SQ, Leung PC, et al. Isolation of anti-inflammatory fractions and compounds from the root of Astragalus membranaceus. Phytother Res. 2013;27(4):581–7. https://doi.org/10.1002/ptr.4759.
Wang DS, Yan LY, Yang DZ, Lyu Y, Fang LH, Wang SB, et al. Formononetin ameliorates myocardial ischemia/reperfusion injury in rats by suppressing the ROS-TXNIP-NLRP3 pathway. Biochem Biophys Res Commun. 2020;525(3):759–66. https://doi.org/10.1016/j.bbrc.2020.02.147.
Fan TJ, Han LH, Cong RS, Liang J. Caspase family proteases and apoptosis. Acta Biochim Biophys Sin (Shanghai). 2005;37(11):719–27. https://doi.org/10.1111/j.1745-7270.2005.00108.x.
Xiong Y, Tang YD, Zheng C. The crosstalk between the caspase family and the cGAS–STING signaling pathway. J Mol Cell Biol. 2021;13(10):739–47. https://doi.org/10.1093/jmcb/mjab071.
Van Opdenbosch N, Lamkanfi M. Caspases in cell death, inflammation, and disease. Immunity. 2019;50(6):1352–64. https://doi.org/10.1016/j.immuni.2019.05.020.
Zhang X, Bi L, Ye Y, Chen J. Formononetin induces apoptosis in PC-3 prostate cancer cells through enhancing the Bax/Bcl-2 ratios and regulating the p38/Akt pathway. Nutr Cancer. 2014;66(4):656–61. https://doi.org/10.1080/01635581.2014.894098.
Zha Q, Zhang L, Guo Y, Bao R, Shi F, Shi Y. Preparation and study of folate modified albumin targeting microspheres. J Oncol. 2022;2022:3968403. https://doi.org/10.1155/2022/3968403.
Wang L, Wang YS, Chen RY, Feng CL, Wang H, Zhu XW, et al. PLGA microspheres as a delivery vehicle for sustained release of tetracycline: biodistribution in mice after subcutaneous administration. J Drug Deliv Sci Technol. 2013;23(6):547–53. https://doi.org/10.1016/s1773-2247(13)50083-9.
Lv S, Jing R, Liu X, Shi H, Shi Y, Wang X, et al. One-step microfluidic fabrication of multi-responsive liposomes for targeted delivery of doxorubicin synergism with photothermal effect. Int J Nanomedicine. 2021;16:7759–72. https://doi.org/10.2147/ijn.S329621.
Cao X, Liu Q, Shi W, Liu K, Deng T, Weng X, et al. Microfluidic fabricated bisdemethoxycurcumin thermosensitive liposome with enhanced antitumor effect. Int J Pharm. 2023;641:123039. https://doi.org/10.1016/j.ijpharm.2023.123039.
Schuster B, Junkin M, Kashaf SS, Romero-Calvo I, Kirby K, Matthews J, et al. Automated microfluidic platform for dynamic and combinatorial drug screening of tumor organoids. Nat Commun. 2020;11(1):5271. https://doi.org/10.1038/s41467-020-19058-4.
Nguyen HQ, Seo TS. A 3D printed size-tunable flow-focusing droplet microdevice to produce cell-laden hydrogel microspheres. Anal Chim Acta. 2022;1192:339344. https://doi.org/10.1016/j.aca.2021.339344.
Liu H, Singh RP, Zhang Z, Han X, Liu Y, Hu L. Microfluidic assembly: an innovative tool for the encapsulation, protection, and controlled release of nutraceuticals. J Agric Food Chem. 2021;69(10):2936–49. https://doi.org/10.1021/acs.jafc.0c05395.
Rajput MS, Agrawal P. Microspheres in cancer therapy. Indian J Cancer. 2010;47(4):458–68. https://doi.org/10.4103/0019-509x.73547.
Hu C, He Y. Formononetin inhibits non-small cell lung cancer proliferation via regulation of mir-27a-3p through p53 pathway. Oncologie. 2021;23(2):241–50.
Yu X, Gao F, Li W, Zhou L, Liu W, Li M. Formononetin inhibits tumor growth by suppression of EGFR-Akt-Mcl-1 axis in non-small cell lung cancer. J Exp Clin Cancer Res. 2020;39(1):62. https://doi.org/10.1186/s13046-020-01566-2.
Acknowledgements
The authors thank the Ethics Committee of University for the guidance on animal experiments. The authors also thank the Institute of Pharmacy, Jiangsu University, Zhenjiang, Jiangsu, China for the necessary facilities support to generate the manuscript.
Funding
This work was funded by the National Key R&D Program of China (2018YFE0208600), Key planning social development projects of Zhenjiang in Jiangsu Province (SH2021024), National Natural Science Foundation of China (81720108030 and 82173785), Natural Science Foundation of the Higher Education Institutions of Jiangsu Province (18KJB360001), Natural Science Foundation of Jiangsu Province (BK20180866), and Postdoctoral Research Fund of Jiangsu Province in 2021 category A (2021K010A).
Author information
Authors and Affiliations
Contributions
XC, XX, and QW: conceptualization. QL, QL, TD, and XW: data curation and methodology. QL, XL, and KL: formal analysis and validation. QY, WD, and GX: supervision. XC, QL, QW, and XG: writing of the manuscript. XC, JY, and XX: funding acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information

Fig. S1
Optimization of microsphere prescription. A, Histogram of particle size distribution at different levels of factors PLGA (0%, 0.5%, 2%) and the corresponding level microscope. B, Histogram of particle size distribution at different levels of factor PEGDA (0%, 5%, 10%) and the corresponding level microscope. C, Histogram of particle size distribution at different levels (0%, 1%, 2%) of factor PI and the corresponding microscope images. D, Histogram of particle size distribution at different levels (0%, 1%, 3%) of factor PVA and the corresponding microscope images. (mean ± SD, n = 100) (PNG 966 kb)

Fig. S2
A chemistry illustration for the crosslinking reaction of PEGDA with PI under UV light (PNG 55 kb)

Fig. S3
Cumulative release curve of free formononetin and formononetin-loaded microsphere in pH 5.0 buffer solutions containing 1% Tween 80 at 37°C (A, 0 d–7 d; B, 8 d–14 d; C, 15 d–21 d; D, 22 d–28 d; E, 29 d–35 d) (PNG 128 kb)

Fig. S4
Cumulative release curve of free formononetin and formononetin-loaded microsphere in pH 6.8 buffer solutions containing 1% Tween 80 at 37°C. (A, 0 d–7 d; B, 8 d–14 d; C, 15 d–21 d; D, 22 d–28 d; E, 29 d–35 d) (PNG 142 kb)

Fig. S5
Cumulative release curve of free formononetin and formononetin-loaded microsphere in pH 7.4 buffer solutions containing 1% Tween 80 at 37°C (A, 0 d–7 d; B, 8 d–14 d; C, 15 d–21 d; D, 22 d–28 d; E, 29 d–35 d) (PNG 141 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cao, X., Li, Q., Li, X. et al. Enhancing Anticancer Efficacy of Formononetin Microspheres via Microfluidic Fabrication. AAPS PharmSciTech 24, 241 (2023). https://doi.org/10.1208/s12249-023-02691-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02691-9