Abstract
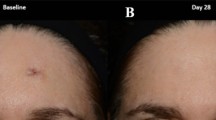
Common warts are benign skin lesions caused by the human papillomavirus. Although they are usually not harmful, they can cause pain, depending on their location. While many modalities are available for treatment of warts, none is a gold standard, and many are not affordable and/or have suboptimal outcomes. Trichloroacetic acid (TCA) is a chemical tissue-destroying agent used as a highly concentrated solution for wart management. While available and efficient, it is difficult to handle as the solution spreads to tissue surrounding the wart causing pain and burning. Hence, we developed a new polymer-based gel of high TCA content (100% w/v). Gels were formed successfully as hydroxyethyl cellulose (HEC) and chitosan were used to impart viscosity and bioadhesion. Formulae of different concentrations were tested for their physical properties, and the optimal formulation was selected for clinical evaluation. A combination of 3% HEC and 2% chitosan provided optimal viscosity and limited water content and have acceptable stability. The efficacy and safety of the biweekly application of TCA gel were evaluated in 30 patients. The clinical study revealed gel’s efficacy and tolerability; half of the patients showed a complete cure, and 90% showed improvement within 6 weeks. Only 10–12% of the patients reported side effects. In summary, transforming TCA solution into a gel enabled its application and handling in a practical manner by physicians and patients alike, while maintaining its efficacy as a tissue-destroying agent. Moreover, it is economic and easy to apply, rendering it a promising formulation for similar conditions requiring controlled tissue ablation.
Graphical Abstract







Similar content being viewed by others
References
Sterling JC, Gibbs S, Haque Hussain SS, Mohd Mustapa MF, Handfield-Jones SE. British Association of Dermatologists’ guidelines for the management of cutaneous warts 2014. Br J Dermatol. 2014;171(4):696–712. https://doi.org/10.1111/bjd.13310.
Ting Y, Manos MM. Detection and typing of genital human papillomaviruses. New York: Academic; 1990.
Mattei P, Nichol PF, Rollins I, Muratore CS. Fundamentals of pediatric surgery. Springer; 2017.
Abdel Meguid AM, Abdel Motaleb AA, Abdel Sadek AMI. Cryotherapy vs trichloroacetic acid 90% in treatment of common warts. J Cosmet Dermatol. 2019;18(2):608–13. https://doi.org/10.1111/jocd.12805.
El-Mohamady AE-S, Mearag I, El-Khalawany M, Elshahed A, Shokeir H, Mahmoud A. Pulsed dye laser versus Nd:YAG laser in the treatment of plantar warts: a comparative study. Lasers Med Sci. 2014;29(3):1111–6. https://doi.org/10.1007/s10103-013-1479-y.
Mulhem E, Pinelis S. Treatment of nongenital cutaneous warts. Am Fam Physician. 2011;84(3):288–93.
Cockayne S, Hewitt C, Hicks K, Jayakody S, Kang’ombe AR, Stamuli E, et al. Cryotherapy versus salicylic acid for the treatment of plantar warts (verrucae): a randomised controlled trial. BMJ. 2011;342:d3271. https://doi.org/10.1136/bmj.d3271.
Lipke MM. An armamentarium of wart treatments. Clin Med Res. 2006;4(4):273–93. https://doi.org/10.3121/cmr.4.4.273.
PubChem, 2022. https://pubchem.ncbi.nlm.nih.gov/compound/Trichloroacetic-acid. Accessed 3 July 2022.
Nguyen AT, Ahmad J, Fagien S, Rohrich RJ. Cosmetic medicine: facial resurfacing and injectables. Plast Reconstr Surg. 2012;129(1):142e–53e. https://doi.org/10.1097/PRS.0b013e3182362c63.
Small R, O’Hanlon K. 22 - Chemical peels. In: Usatine RP, Pfenninger JL, Stulberg DL, Small R, editors. Dermatologic and cosmetic procedures in office practice. Philadelphia: W.B. Saunders; 2012. p. 259–73.
Abdellah MS, Elsaman AM. Trichloroacetic acid for the treatment of dysfunctional uterine bleeding: a pilot prospective clinical trial. Eur J Obstet Gynecol Reprod Biol. 2012;165(2):280–3. https://doi.org/10.1016/j.ejogrb.2012.07.003.
Kopera D, Holubar K. Trichloroacetic acid in dermatology of 1911. Int J Dermatol. 1998;37(3):205.
Pezeshkpoor F, Banihashemi M, Yazdanpanah MJ, Yousefzadeh H, Sharghi M, Hoseinzadeh H. Comparative study of topical 80% trichloroacetic acid with 35% trichloroacetic acid in the treatment of the common wart. J Drugs Dermatol. 2012;11(11):e66–9.
Bodar P, Agarwal P, Saikia S, Dalal T, Jagati A. Evaluating the efficacy of 100% trichloroacetic acid needling in the treatment of palmoplantar warts. Indian J Drugs Dermatol. 2020;6(1):13–6. https://doi.org/10.4103/ijdd.ijdd_38_19.
Baker GE, Tyring SK. Therapeutic approaches to papillomavirus infections. Dermatol Clin. 1997;15(2):331–40. https://doi.org/10.1016/s0733-8635(05)70441-1.
Zanini M. Trichloroacetic acid gel-a new method for an old acid. Med Cutan Ibero Lat Am. 2007;35(1):14–7.
Hendriks M, Bouter P, Kolodziek K. Composition for the treatment of skin and/or nail lesions. EP2407151B1(Patent) 2012.
Wedmore I, McManus JG, Pusateri AE, Holcomb JB. A special report on the chitosan-based hemostatic dressing: experience in current combat operations. J Trauma. 2006;60(3):655–8. https://doi.org/10.1097/01.ta.0000199392.91772.44.
El Fawal G, Hong H, Song X, Wu J, Sun M, He C, et al. Fabrication of antimicrobial films based on hydroxyethylcellulose and ZnO for food packaging application. Food Packag Shelf Life. 2020;23:100462. https://doi.org/10.1016/j.fpsl.2020.100462.
Rao KS, Naidu V, Savitha K. Cryosurgery vs 40 % salicylic acid in treatment of warts. Medica Innovatica. 2014;3(1):65–71.
Pawlak A, Mucha M. Thermogravimetric and FTIR studies of chitosan blends. Thermochim Acta. 2003;396(1):153–66. https://doi.org/10.1016/S0040-6031(02)00523-3.
Ayouch I, Kassem I, Kassab Z, Barrak I, Barhoun A, Jacquemin J, et al. Crosslinked carboxymethyl cellulose-hydroxyethyl cellulose hydrogel films for adsorption of cadmium and methylene blue from aqueous solutions. Surf Interfaces. 2021;24:101124. https://doi.org/10.1016/j.surfin.2021.101124.
Angadi SC, Manjeshwar LS, Aminabhavi TM. Interpenetrating polymer network blend microspheres of chitosan and hydroxyethyl cellulose for controlled release of isoniazid. Int J Biol Macromol. 2010;47(2):171–9. https://doi.org/10.1016/j.ijbiomac.2010.05.003.
Lustriane C, Dwivany FM, Suendo V, Reza M. Effect of chitosan and chitosan-nanoparticles on post harvest quality of banana fruits. J Plant Biotechnol. 2018;45(1):36–44. https://doi.org/10.5010/JPB.2018.45.1.036.
Gorgieva S, Kokol V. Synthesis and application of new temperature-responsive hydrogels based on carboxymethyl and hydroxyethyl cellulose derivatives for the functional finishing of cotton knitwear. Carbohyd Polym. 2011;85(3):664–73. https://doi.org/10.1016/j.carbpol.2011.03.037.
Gelaw TB, Sarojini BK, Kodoth AK. Chitosan/hydroxyethyl cellulose gel immobilized polyaniline/CuO/ZnO adsorptive-photocatalytic hybrid nanocomposite for Congo red removal. J Polym Environ. 2022;30(10):4086–101. https://doi.org/10.1007/s10924-022-02492-4.
Hezaveh H, Muhamad II. Effect of natural cross-linker on swelling and structural stability of kappa-carrageenan/hydroxyethyl cellulose pH-sensitive hydrogels. Korean J Chem Eng. 2012;29(11):1647–55. https://doi.org/10.1007/s11814-012-0056-6.
Strokova NE, Savilov SV, Morozov II, Yagodovskaya TV, Lunin VV. Laboratory simulations of the interaction between ozone and chloroacetic acids in the conditions close to stratospheric. Russ J Phys Chem A. 2015;89(1):28–37. https://doi.org/10.1134/S0036024415010264.
Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Rev Mater. 2016;1(12):16071. https://doi.org/10.1038/natrevmats.2016.71.
Abd Ellah NH, Abd El-Aziz FE-ZA, Abouelmagd SA, Abd El-Hamid BN, Hetta HF. Spidroin in carbopol-based gel promotes wound healing in earthworm’s skin model. Drug Dev Res. 2019;80(8):1051–61. https://doi.org/10.1002/ddr.21583.
Helmy AM, Elsabahy M, Soliman GM, Mahmoud MA, Ibrahim EA. Development and in vivo evaluation of chitosan beads for the colonic delivery of azathioprine for treatment of inflammatory bowel disease. Eur J Pharm Sci. 2017;109:269–79. https://doi.org/10.1016/j.ejps.2017.08.025.
Fu J, Yang F, Guo Z. The chitosan hydrogels: from structure to function. New J Chem. 2018;42(21):17162–80. https://doi.org/10.1039/C8NJ03482F.
Abouelmagd SA, Ku YJ, Yeo Y. Low molecular weight chitosan-coated polymeric nanoparticles for sustained and pH-sensitive delivery of paclitaxel. J Drug Target. 2015;23(7–8):725–35. https://doi.org/10.3109/1061186X.2015.1054829.
da Silva JB, Dos Santos RS, Vecchi CF, Bruschi ML. Drug delivery platforms containing thermoresponsive polymers and mucoadhesive cellulose derivatives: state of the art and review of patents. Recent Adv Drug Deliv Formul. 2022. https://doi.org/10.2174/2667387816666220404123625.
Postoy V, Kukhtenko H, Vyshnevska L, Gladukh I, Semchenko K. Study of rheological behaviour of hydroxyethyl cellulose gels in the development of the composition and technology of the medicine with anti-inflammatory activity. Pharmacia. 2019;66(4). https://doi.org/10.3897/pharmacia.66.e37267.
Syed TA, Qureshi ZA, Ahmad SA, Ali SM. Management of intravaginal warts in women with 5-fluorouracil (1%) in vaginal hydrophilic gel: a placebo-controlled double-blind study. Int J STD AIDS. 2000;11(6):371–4. https://doi.org/10.1258/0956462001916074.
Bonatti H, Aigner F, De Clercq E, Boesmueller C, Widschwendner A, Larcher C, et al. Local administration of cidofovir for human papilloma virus associated skin lesions in transplant recipients. Transpl Int. 2007;20(3):238–46. https://doi.org/10.1111/j.1432-2277.2006.00430.x.
Abouhussein D, El Nabarawi MA, Shalaby SH, El-Bary AA. Cetylpyridinium chloride chitosan blended mucoadhesive buccal films for treatment of pediatric oral diseases. J Drug Deliv Sci Technol. 2020;57:101676. https://doi.org/10.1016/j.jddst.2020.101676.
Qian L. Cellulose-based composite hydrogels: preparation, structures, and applications. In: Mondal MIH, editor. Cellulose-based superabsorbent hydrogels. Cham: Springer International Publishing; 2019. p. 655–704.
Li J, Xu Z. Physical characterization of a chitosan-based hydrogel delivery system. J Pharm Sci. 2002;91(7):1669–77. https://doi.org/10.1002/jps.10157.
Boufas S, Benhamza MEH, Seghir BB, Hadria F. Synthesis and characterization of chitosan/carrageenan/hydroxyethyl cellulose blended gels. Asian J Res Chem. 2020;13(3):209–15.
Shen X, Shamshina JL, Berton P, Gurau G, Rogers RD. Hydrogels based on cellulose and chitin: fabrication, properties, and applications. Green Chem. 2016;18(1):53–75. https://doi.org/10.1039/C5GC02396C.
Yan S, Yin J, Tang L, Chen X. Novel physically crosslinked hydrogels of carboxymethyl chitosan and cellulose ethers: structure and controlled drug release behavior. J Appl Polym Sci. 2011;119(4):2350–8. https://doi.org/10.1002/app.32678.
Muzio G, Massone C, Rebora A. Treatment of non-genital warts with topical imiquimod 5% cream. Eur J Dermatol. 2002;12(4):347–9.
Bhat RM, Vidya K, Kamath G. Topical formic acid puncture technique for the treatment of common warts. Int J Dermatol. 2001;40(6):415–9. https://doi.org/10.1046/j.1365-4362.2001.01242.x.
Hirose R, Hori M, Shukuwa T, Udono M, Yamada M, Koide T, et al. Topical treatment of resistant warts with glutaraldehyde. J Dermatol. 1994;21(4):248–53. https://doi.org/10.1111/j.1346-8138.1994.tb01731.x.
Khaled A, Ben Romdhane S, Kharfi M, Zeglaoui F, Fazaa B, Kamoun MR. Assessment of cryotherapy by liquid nitrogen in the treatment of hand and feet warts. Tunis Med. 2009;87(10):690–2.
Author information
Authors and Affiliations
Contributions
A.M.H and S.A.A. were responsible for formulation, gel evaluation, and data analysis. S.S.A. and H.M.A. were responsible for protocol development, patient enrollment, clinical data collection, and analysis. R.M.E. contributed to clinical data collection and analysis. First draft of the manuscript was written by S.A.A and H.M.A. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Helmy, A.M., Ahmed, S.S., Sabaa, R.M.E. et al. Towards Formulation of Highly Acidic Active Ingredients: Development of Clinically Effective Concentrated Trichloroacetic Acid Gel for Wart Management. AAPS PharmSciTech 24, 160 (2023). https://doi.org/10.1208/s12249-023-02615-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02615-7