Abstract
Albendazole is a broad-spectrum anthelmintic drug used for parasitic infections. In addition, due to its mechanism of action, it has been studied as an anticancer agent. However, poor and highly variable bioavailability are limiting factors for its use in systemic illnesses. The present study aimed to develop two parenteral formulations of albendazole and to compare its pharmacokinetic profile with the conventional oral administration. Parenteral formulations were developed using two different approaches: a phosphonooxymethylated prodrug and cosolvents. For the albendazole prodrug, once synthetized, its solubility and hydrolysis with alkaline phosphatase were evaluated. A factorial design of experiments was used for the cosolvent formulation. Stability and hemolytic activity were assessed. A pharmacokinetic study was performed on New Zealand rabbits. Both formulations were administered intravenously, and the prodrug was also administered intramuscularly. Results were compared with those obtained after the oral administration of albendazole. A 20,000-fold and 6000-fold increase in albendazole solubility was found with the prodrug and cosolvent formulations, respectively. Both parenteral formulations displayed higher albendazole plasma concentrations for the first 2 h compared with oral administration, even when the oral dose was doubled. The absolute bioavailability of oral albendazole was 15.5% while for the intramuscular administration of the prodrug was 102.6%. Both parenteral formulations showed a significant decrease in the formation of albendazole sulfoxide (ANOVA p<0.05) and allowed greater exposure to albendazole. Albendazole cosolvent parenteral formulation could be a promising option in systemic illnesses considering its ease of preparation and superb pharmacokinetic performance.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Albendazole (ABZ), a benzimidazole derivative approved in 1982 as an anthelmintic product, is the drug of choice for cystic hydatid disease as well as for the treatment of parenchymal neurocysticercosis [1]. Its mechanism of action is related to its inhibitory effect on tubulin polymerization, which results in the selective degeneration of cytoplasmic microtubules and the death of the parasite [2].
Due to this effect, ABZ has also been evaluated for its potential against different types of cancer cells including liver [3, 4], stomach [5], colon [6], pancreas [7], ovarian [8], and leukemia [9]. A limiting factor in albendazole dosage regimes is its low solubility which leads to a great interindividual variability in plasma concentrations [2].
Although the oral administration is the most common for the convenience of the patients, the parenteral route represents a good option to attain the desired plasma concentrations. Few studies have described the development of ABZ parenteral formulations. In 2015, Noorani et al. assembled ABZ with bovine serum albumin into nanoparticles (nab) and reported a significant improvement in solubility and better efficacy than free ABZ in an ovarian cancer xenograft model [10]. Pillai K. et al. reported a new parenteral formulation, using sulfobutylether-β-cyclodextrin. Results showed that the solubility of ABZ in the complexed product was 8 mg/mL. Also, a potent cytotoxic in vitro and in vivo effect was found in an ovarian cancer cell line and in a BALB/c mice murine model with an ovarian tumor [11]. Recently, Viranov et al. evaluated the effect of different surfactants on ABZ solubility and found that the most promising candidate for parenteral application was a mixture of 60% of sodium dioctyl sulfosuccinate and 40% of the phospholipid sodium dipalmitoyl-phosphatidylglycerol. In this delivery system, a solubility of 4.4 mg/mL of ABZ was found [12].
Although the abovementioned strategies have improved ABZ bioavailability, most of them have some limitations. For instance, the size of the nab nanoparticles may limit the access of the drug into the tumor [10]. In the case of using surfactants, the need to use acidic conditions (pH=3) is a limitation because it might cause local irritation in parenteral administration [12].
Other alternatives to increase the drug solubility are the formation of prodrugs and the use of cosolvents. For example, Chassaing et al. developed phosphonooxymethylated prodrugs of benzimidazoles. This study showed a 195,000-fold increase in the solubility of the fenbendazole prodrug. Also, higher plasma concentrations were obtained after the oral administration of the prodrug compared with the administration of an oral suspension [13].
In the case of cosolvents, when drugs are lipophilic or nonpolar and have low water solubility, they can be solubilized using aqueous solutions and water-miscible organic solvents (cosolvents). If this strategy does not allow to reach the adequate concentration of the drug, it is possible to use nonaqueous solutions composed of cosolvents [14]. The advantages of liquid cosolvent formulations include the following: no need for expensive technology for producing the dosage form, easy sterilization process, and facility in the administration process [15].
In the current study, we developed and evaluated two parenteral formulations of ABZ to improve its solubility and bioavailability. Techniques employed for the formulations were a phosphonooxymethylated prodrug approach and the use of cosolvents.
Materials and Methods
Materials
Albendazole analytical standard, 1,4-dioxane, 4 M HCl in dioxane, cesium carbonate (Cs2CO3), polyethylene glycol 400 (PEG 400), formic acid, and alkaline phosphatase EC 3.1.3.1 (type VII-S: from bovine intestinal mucosa) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The albendazole sulfoxide (ABZSO) analytical standard was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Dimethylformamide (DMF), dimethylacetamide (DMA), methanol, dimethyl sulfoxide (DMSO), dichloromethane (DCM), ethyl ether, chloroform, ethanol (EtOH), acetonitrile, sodium bicarbonate (NaHCO3), sodium sulfate (Na2SO4), sodium hydroxide (NaOH), ammonium dihydrogen phosphate (NH4H2PO4), and sodium benzoate were purchased from J. T Baker (PA, USA). Isopropyl myristate, glycerin, castor oil, cremophor EL, propylene glycol (PG), and polyethylene glycol 600 (PEG 600) were kindly provided by Gattefossé (Saint-Priest Cedex, France). Di-tert-butyl (chloromethyl) phosphate was purchased from AccelaChemBio Co. (Pudong New Area, Shanghai).
Distilled or deionized water was obtained from the Elix 3 and Milli-Q water purification system (Millipore, Milford, MA, USA). Solvents used for chromatographic analysis were HPLC grade. All other reagents and solvents were of analytical grade.
Preparation of Phosphonooxymethylated ABZ Prodrug
The ABZ prodrug was prepared according to the synthetic sequence shown in Fig. 1. The synthesis was based on the methodologies previously reported by Chassaing et al. [13] and Flores et al. [16].
Preparation of Methyl (1-(((di-tert-butoxyphosphoryl)oxy)methyl)-6-(propylthio)-1H-benzimidazol-2-yl)carbamate (3) from ABZ (1)
An ABZ (1) suspension in 25 mL of anhydrous DMF (1.0062 g, 3.77 mmol) was prepared, and Cs2CO3 (1.8700 g, 5.74 mmol) was added and stirred for 3 h at room temperature. Di-tert-butyl(chloromethyl) phosphate (1.464 g, 5.66 mmol) was dissolved in anhydrous DMF in an ice bath; then the ABZ mixture previously prepared was added dropwise while stirring and maintained at 0°C for 1 h. The reaction was stirred at room temperature for 6 h. DCM (400 mL) was added, and the reaction mixture was washed with 2% NaHCO3 (3 × 300 mL). A final washing was performed using water (300 mL). The organic phase was dried over Na2SO4, filtered, and volatiles were evaporated. The residue (containing compound 3) was used without purification in the following step.
Preparation of Methyl (1-((phosphonooxy)methyl)-6-(propylthio)-1H-benzimidazol-2-yl)carbamate (5)
The residue (containing compound 3) dissolved in 1,4-dioxane (10 mL) was cooled in an ice bath and treated with a 4 M solution of HCl in 1,4-dioxane (15 mL) by adding it dropwise. The mixture was stirred for 2 h at room temperature; then the solvent was eliminated under vacuum to afford compound (5).
Preparation of Sodium (2-((methoxycarbonyl)amino)-6-(propylthio)-1H-benzimidazol-1-yl)methyl phosphate (6)
The crude compound (5) was dissolved in MeOH (7 mL). Then, a solution of NaOH in MeOH:water (6:3) was added dropwise to reach pH 7 while stirring. Volatile solvents were removed on a rotary evaporator, and the solid residue was suspended in anhydrous EtOH with stirring for 3 h. The solid (6) was filtered, re-suspended, and maintained at stirring for 24 h in DCM to remove residual ABZ and other non-polar compounds. The solid obtained was placed in MeOH under continuous stirring for 24 h to dissolve the prodrug. The solvent was evaporated under a vacuum, and the solid recovered was dried in a vacuum for 72 h. The compound was characterized by 1H NMR, 13C NMR, 31P NMR, and MS data. Th residual content of solvents (DCM and dioxane) was determined by quantitative 1H NMR using chloroacetic acid as an internal standard.
Solubility of the Prodrug
Solubility was evaluated in buffer solutions at pH 6.0, 7.4, and 8.0 using the shake flask method. Briefly, an excess amount of the compound was added to each solution within a water bath maintained at 25 +1°C. The tubes were kept on an oscillatory shaker at 150 rpm for 48 h. Then, they were filtered and diluted with water. Concentrations were determined by HPLC (Shimadzu LC-10ATvp) using a Spherisorb ODS1 (5μm, 250 × 4.6 mm) column. The mobile phase was 1% NH4H2PO4:methanol:acetonitrile (30:50:20 v/v) at a flow rate of 1 mL/min and a UV detector at 245 nm. Samples were prepared in triplicates.
Hydrolysis of the Prodrug
One milligram of prodrug was dissolved in 1.2 mL of Tris/HCl buffer pH 8.5 and placed in a water bath at 37°C. After 10 min, 50 μL (88 units) of alkaline phosphatase was added. Samples were withdrawn at 10, 20, and 30 s and 1, 3, 5, 10, and 90 min and mixed with 900 μL of acetonitrile to stop the enzymatic activity. Samples were centrifuged for 10 min at 14,000 rpm. The supernatant was analyzed for ABZ formation by HPLC.
Development of a Formulation Using Cosolvent Approach
Solubility of ABZ in isopropyl myristate, glycerin, castor oil, cremophor EL, PG, ethanol, PEG 600, PEG 400, DMSO, and DMA was evaluated using the shake flask method. For the assay of ABZ, samples were diluted with the mobile phase, and 30 μL was injected into the chromatographic system (Shimadzu LC-10ATvp), using an Hypersil ODS column (5μm, 150 × 4.6 mm), a mobile phase consisting of 1.25% NH4H2PO4:methanol:acetonitrile (30:50:20 v/v), a flow rate of 1.2 mL/min, and a UV detector at 295 nm. The method was linear in the range of 3.0–25.0 μg/mL.
According to ABZ solubility, the solvents selected were DMA, PEG 400, and EtOH. To choose the proportion of each solvent, a 32 factorial design of experiments (DOE) was used. DOE was carried out using Statgraphics Centurion XVI version 16.1.18. The response variable was ABZ solubility. The formulation with the highest ABZ concentration was named F1.
To reduce hemolytic potential, different proportions of EtOH (60, 70, and 80% v/v) and 6% sodium benzoate were used. The formulation with the highest ABZ concentration was named F2.
Physical and Chemical Stability
Formulations F1 and F2 were prepared at an ABZ concentration of 5 mg/mL. Formulations were stored at room temperature and refrigerated at 4°C. Samples were analyzed after they were prepared, and at 6 and 12 months for drug content, pH, and physical appearance.
Hemolysis Assay
Hemolysis of both formulations was determined in fresh rabbit blood. Negative and positive controls were 0.9% saline solution and 1% Triton X100, respectively. Briefly, blood was centrifuged at 3500 rpm for 10 min. Blood cells were separated and washed with 0.9% saline solution. Cells were suspended in saline solution, an aliquot of 0.9 mL was placed in a water bath at 37°C, and 0.1 mL of ABZ formulations or control solutions was added, vortexed for 5 s, and maintained at 37°C for 2 min. Samples were centrifuged at 3500 rpm for 10 min. A 0.3 mL aliquot of the supernatant was mixed with 5.7 mL of saline solution, and absorbance was measured at 540 nm. The percentage of hemolysis was calculated as:
Pharmacokinetic Study
Animal experiments were approved by the Institutional Animal Care and Use Committee (CICUAL) of the Chemistry Faculty, UNAM. Protocol was approved on August 16, 2021, with the official number FQ/CICUAL/435/21. All experiments were conducted according to Mexican Guidance (NOM-062-ZOO-1999).
A pharmacokinetic study was performed in twelve male white New Zealand rabbits (2.5–3.5 kg). The animals were individually housed with access to food and water ad libitum. Rabbits were randomly assigned to two different groups. Group A received an oral dose of 0.8 mL of Zentel® suspension (40 mg ABZ/mL) while group B received 1 mL of the aqueous solution of the prodrug intramuscularly at a concentration equivalent to 15.88 mg/mL of ABZ. After a washout period of 15 days, group A received 3 mL of formulation F2 intravenously (5 mg/mL) in the marginal ear vein, and group B received 1 mL of the prodrug intravenously at a concentration equivalent to 15.88 mg/mL of ABZ. Immediately prior to administration, both parenteral formulations were filtered through 0.22-μm nylon filters.
Blood samples were taken from the marginal ear vein prior to dosing (0 h) and at 20 and 40 min and 1, 1.5, 2, 3.5, 5, 8, 12, and 24 h post administration. In the parenteral administration, a sampling time of 10 min was added. Samples were collected on EDTA K2 tubes and centrifuged. Plasma samples were stored at −20°C until analysis.
Albendazole and Albendazole Sulfoxide Assay
For the assay of ABZ and ABZSO, a liquid chromatographic method coupled with tandem mass spectrometry (LC-MS/ MS) was developed and validated using a Shimadzu Prominence chromatograph (Shimadzu, Japan) coupled to a turbo ion spray ionization-triple quadrupole mass spectrometer, API 4500 (ABSciex, Darmstadt, Germany), with positive ion electrospray ionization using multiple reaction monitoring (MRM) mode to detect and quantify ABZ and ABZSO with mass-to-charge (m/z) transitions at 266.3 → 234.0 and 282.0 → 240.0, respectively.
Briefly, plasma samples (200 μL) were spiked with 10 μL of internal standard (carbamazepine, 2000 ng/mL). After vortex mixing, 3 mL of ether:dichloromethane:chloroform (60:30:10 v/v) was added. Samples were mixed and centrifuged at 3500 rpm for 10 min at 4°C. The organic phase was evaporated to dryness with a nitrogen stream. The residue was reconstituted with 200 μL of mobile phase, and 1 μL was injected into the chromatographic system, using a Sepax HP-C18 (5μm, 150 × 4.5 mm) column and an HP-C18 (5μm, 10 × 4 mm) precolumn. The mobile phase was methanol:0.1% formic acid (70:30, v/v) at a flow rate of 0.7 mL/min.
Pharmacokinetic Analysis
Pharmacokinetic parameters of ABZ and ABZSO were estimated using Phoenix® WinNonlin® Version 8.3.3.33 (Pharsight, Mountain View, CA, USA). To compare the relative formation of ABZSO, the individual ABZSO/ABZ AUC0–∞ ratio for each administration was calculated, and the mean ratios were compared. Also, the absolute bioavailability was determined for both extravascular administrations.
Statistical Analysis
ANOVA statistical comparisons were performed using Statgraphics Centurion XVI version 16.1.18 followed by a Tukey test.
Results
Preparation of Phosphonooxymethylated Prodrug of ABZ
The prodrug was obtained through an alkylation of ABZ employing di-tert-butyl(chloromethyl) phosphate in DMF. A strong base was used to create the nucleophile (2) (Fig. 1). In the first instance, we proposed sodium hydride as the strong base; however, it promoted the formation of a methylene-bis-albendazole (4), which was the most abundant secondary product [13]. This compound (4) was isolated and characterized by 1H NMR. A characteristic signal was obtained around 6.5 ppm, which was attributed to methylene binding albendazole molecules in the dimer.
To avoid the formation of compound (4), we decided to look for a weaker inorganic base in the same solvent. Cesium carbonate was selected because it has been used in similar reactions with an adequate performance [17]. When this reactive was used, the quantity of dimer was decreased; nevertheless, ABZ maintained a low reactivity. To achieve better yields, several reaction conditions including temperature, the quantity of electrophilic reagent, and the use of a Finkelstein activator as KI were tested [17]. The best results were obtained with the conditions mentioned in the methodology section.
A column purification of compound (3) was attempted, but ABZ appeared in all fractions. The only way to separate it was to use an acid-mobile phase; however, this led to the formation of compound (5), which was strongly retained on the column. Therefore, we decided to continue with the other reactions without purification.
Once compound (3) was obtained; an acid-promoted deprotection allowed us to obtain prodrug (5). Finally, the conversion of the free acid to the corresponding disodium salt was achieved with NaOH in methanol. These last two reactions were quick and did not require further optimization. However, the purification of the final product was complicated because most of the impurities were soluble in the same solvents as the compound (6). It is important to note that the prodrug was obtained as a mixture of their N1 and N3 regioisomers; however, this fact is irrelevant since both are enzymatically hydrolyzed to give ABZ. The global yield was 11%. Spectroscopical characterization of ABZ prodrug is included in the Supplementary material. Mass (Figure S1) and NMR spectra (Figures S2–S4) showed results for compound (5), since compound (6) when dissolved in water produces compound (5). Analysis of residual solvents using 1H NMR spectra showed that dichloromethane was not detected. In the case of dioxane, the concentration was 224 ppm, which is lower than the ICH guidance specification (< 380 ppm) [18].
Prodrug Solubility
As shown in Table I, the prodrug solubility was independent of pH in the range evaluated. A 20,000-fold increase in solubility was found, when compared to the reported ABZ solubility in water of 0.8 mg/L (3×10−3 mM) [19].
Hydrolysis of the Prodrug
ABZ was rapidly hydrolyzed in an alkaline phosphatase solution with a first-order half-life of 8.72 ± 0.23 min. Since the hydrolysis of both regioisomers resulted in the same active compound, the pharmacokinetic study was performed with a mixture of them.
Development of a Formulation Using Cosolvent Approach
Table II shows the ABZ solubility in the different cosolvents evaluated. Based on these results and considering the toxicity and hemolytic potential reported, the cosolvents selected were DMA, PEG 400, and EtOH. Table III shows the characteristics of the DOE. Different DMA and PEG 400 proportions were evaluated while the EtOH proportion was fixed at 25% v/v. Figure 2 shows the response surface plot of DOE. It was found that the highest ABZ solubility was reached with the high level of both solvents. From these results, the formulation named F1, composed of 30% v/v DMA, 50% w/v PEG 400, and 25% v/v of EtOH was selected. The concentrations used complied with the FDA requirements for intravenous formulations [20].
When the different proportions of EtOH and sodium benzoate were evaluated, it was found that the highest solubility was achieved using 30% v/v DMA, 50% w/v PEG 400, and 25% v/v of sodium benzoate 6% in ethanol 70% v/v, and therefore, this formulation, named F2, was selected. Formulations, F1 and F2, were prepared at an ABZ concentration of 5 mg/mL for further studies.
Physical and Chemical Stability
Both formulations were colorless and transparent at the time of preparation. At room temperature, both formulations maintained their appearance, and no precipitation was observed even after 12 months of storage. Besides, drug content remained between 90 and 110%, which is the limit to be considered stable [21]. The pH was maintained throughout the time interval at a value of approximately 5. On the other hand, under refrigeration conditions, a white precipitate was formed at the end of the sixth month in both formulations, and the evaluation was not continued.
Hemolysis
Figure 3 shows the mean percentage of hemolysis for formulations F1 and F2. The hemolysis was lower than 12%, and therefore, formulations were considered non-hemolytic [22]. Considering that formulation F2 presented the lowest percentage of hemolysis, it was selected for the in vivo study.
Pharmacokinetic Evaluation
Analytical Assay for ABZ and ABZSO
The method was linear using a weight factor of 1/x. The calibration curves were linear in the range of 0.5–1000.0 ng/mL for ABZ and from 10.0 to 1500.0 ng/mL for ABZSO. The method was precise with intra-day coefficients of variation in the range of 2.4–8.0% for ABZ and 1.4–5.3% for ABZSO, while the inter-day assay precision was between 11.5 and 14.6% for ABZ and 2.6% and 4.7% for ABZSO. Accuracy was also demonstrated with deviation from the nominal concentration ranging from 0.3 to 6.2% for ABZ and 1.7 to 9.9% for ABZSO. Precision and accuracy were maintained after sample dilution ratios of 1:2 and 1:5.
Pharmacokinetic Study
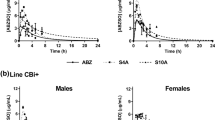
The mean pharmacokinetic profiles of ABZ and ABZSO after different routes of administration are shown in Fig. 4. ABZ concentrations were quantifiable up to 12 h after the dose, while ABZSO concentrations were quantifiable up to 24 h. It is important to consider that the oral dose of ABZ was twice the dose used in the other routes of administration.
The pharmacokinetic parameters of ABZ and ABZSO are presented in Tables IV and V, respectively. There were no statistically significant differences in t1/2 of elimination (p > 0.05) of both analytes regardless of the route of administration. Similarly, no significant differences were found in Vz and Cl of ABZ after the intravenous administration of cosolvent and prodrug formulations.
After Cmax and AUClast dose normalization, it was found that there was a significant difference (p < 0.01) between the parenteral and oral routes (Table IV), being greater the exposure of ABZ in the parenteral routes.
For oral administration, the absolute bioavailability of ABZ was 15.5%. For the prodrug, the absolute bioavailability after intramuscular administration was 102.6% with respect to intravenous administration of the same prodrug.
Discussion
Different parenteral albendazole formulations were developed using two different approaches to improve its solubility. The first technique consisted of the synthesis of a phosphonooxymethylated prodrug of ABZ. This methodology has already been used for other benzimidazoles [13, 23], and even fosphenytoin, a prodrug of phenytoin, has been approved by FDA and is available for parenteral administration [24].
Despite the low yield obtained during the synthesis, the prodrug possesses characteristics that make it a good candidate for a parenteral formulation. First of all, we found an improvement of 20,000-fold in ABZ solubility. In addition, the prodrug was able to rapidly release ABZ using alkaline phosphatase.
The other technique that was selected to improve ABZ solubility was the use of cosolvents. Due to the low solubility of ABZ, a combination of non-aqueous solvents was selected for the cosolvent formulation. This strategy has been used for other low-solubility drugs [14].
Formulations F1 and F2 resulted in a significant 6000-fold increase in ABZ solubility. In addition, both formulations were stable for 12 months at room temperature, and the hemolytic potential was lower than the proposed maximum value of 20% [22].
Pharmacokinetic parameters obtained by the oral administration, which is the only route of administration currently available, showed that t1/2 and tmax of ABZSO were similar to those reported in other studies in rabbits [25, 26]. Different studies have reported the difficulty to quantify ABZ concentrations due to its high and rapid metabolism after the oral route [27,28,29]. To our knowledge, this is the first study that reports ABZ pharmacokinetic parameters, including absolute bioavailability, in a rabbit model after oral administration. Results showed that the half-life of ABZ is relatively shorter than that of ABZSO. In addition, a higher variability in plasma concentrations of ABZ and ABZSO was found after oral administration than in parenteral routes. Furthermore, the Cmax after the oral route was found to be much lower than concentrations after parenteral administrations despite the fact that double the dose was administered, which is consistent with the expected low bioavailability.
By comparing the pharmacokinetics of the intravenous cosolvent formulation vs. the oral suspension, a similar elimination half-life in both routes was found. The pharmacokinetic profile of ABZ (Fig. 4) showed that the intravenous administration maintained a much higher concentration of ABZ than the oral route at least for the first 2 h even when the dose was half the dose of the oral administration.
When the formulations (prodrug and cosolvent) administered by intravenous route were compared, there were no significant differences in any of the pharmacokinetic parameters neither for ABZ nor for ABZSO, demonstrating that in vivo, ABZ was rapidly released from the phosphonooxymethylated prodrug corroborating the in vitro results.
With respect to the prodrug administration by intravenous and intramuscular routes, no changes in pharmacokinetic performance were found. This indicates a very rapid disposition of ABZ from the prodrug. Furthermore, a complete bioavailability of ABZ was obtained (F=102.6%). Due to these observations, the intramuscular route could be considered the route of choice for ABZ prodrug administration considering that it is safer than intravenous administration.
ABZ has been proposed for cancer treatment, and some studies have shown that it has a greater activity than its metabolites [30, 31]. Considering this information, the relative formation of metabolite after each administration route was compared using the ABZSO/ABZ AUC0–inf ratio. The results in Table V indicate that the mean ABZSO/ABZ AUC0–∞ ratio was similar for parenteral administrations. However, a statistical difference was found with the oral route, with higher metabolite formation in the latter.
The lower formation of ABZSO in the parenteral route results in a higher proportion of ABZ, which added to its more lipophilic character [30], and the fact that it is not a substrate of Bcrp1 and P-gp [32] allows the drug to reach its site of action and to penetrate the target cells more easily. The results are promising for the further evaluation of its efficacy in in vivo cancer models.
Conclusion
In the present study, two parenteral formulations of ABZ were developed using prodrug and cosolvent approaches. Both formulations showed a significant improvement in ABZ plasma exposure compared to oral administration. In addition, the parenteral route exhibited a significant decrease in the formation of the ABZSO.
Considering that there were no significant differences in the pharmacokinetic parameters of the parenteral formulations, either of them would be a good choice for the treatment of systemic diseases. However, the cosolvent formulation has the advantage that it does not need expensive technology, which would make it more affordable and thus accessible for the patients in need of such treatment. Taking this into account, this formulation could be suitable for ABZ parenteral administration. More studies should be performed in order to determine the safety and efficacy of the formulation.
Data Availability
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information files. Should any raw data files be needed in another format, they are available from the corresponding author upon reasonable request.
References
Schulz JD, Neodo A, Coulibaly JT, Keiser J. Pharmacokinetics of albendazole, albendazole sulfoxide, and albendazole sulfone determined from plasma, blood, dried-blood spots, and mitra samples of hookworm-infected adolescents. Antimicrob Agents Chemother. 2019;63(4) https://doi.org/10.1128/AAC.02489-18.
Jung-Cook H. Pharmacokinetic variability of anthelmintics: implications for the treatment of neurocysticercosis. Expert Rev Clin Pharmacol. 2012;5:21-30. https://doi.org/10.1586/ecp.11.72.
Rolin S, Souhaili-el Amri H, Batt AM, Levy M, Bagrel D, Siest G. Study of the in vitro bioactivation of albendazole in human liver microsomes and hepatoma cell lines. Cell Biol Toxicol. 1989;5:1-14. https://doi.org/10.1007/BF00141060.
Pourgholami MH, Woon L, Almajd R, Akhter J, Bowery P, Morris DL. In vitro and in vivo suppression of growth of hepatocellular carcinoma cells by albendazole. Cancer Lett. 2001;165:43-9. https://doi.org/10.1016/s0304-3835(01)00382-2.
Zhang X, Zhao J, Gao X, Pei D, Gao C. Anthelmintic drug albendazole arrests human gastric cancer cells at the mitotic phase and induces apoptosis. Exp Ther Med. 2017;13:595-603. https://doi.org/10.3892/etm.2016.3992.
Pourgholami MH, Akhter J, Wang L, Lu Y, Morris DL. Antitumor activity of albendazole against the human colorectal cancer cell line HT-29: in vitro and in a xenograft model of peritoneal carcinomatosis. Cancer Chemother Pharmacol. 2005;55:425-32. https://doi.org/10.1007/s00280-004-0927-6.
Chen H, Weng Z, Xu C. Albendazole suppresses cell proliferation and migration and induces apoptosis in human pancreatic cancer cells. Anticancer Drugs. 2020;31:431-9. https://doi.org/10.1097/CAD.0000000000000914.
Pourgholami MH, Wangoo KT, Morris DL. Albendazole-cyclodextrin complex: enhanced cytotoxicity in ovarian cancer cells. Anticancer Res. 2008;28:2775-9.
Khalilzadeh A, Wangoo KT, Morris DL, Pourgholami MH. Epothilone-paclitaxel resistant leukemic cells CEM/dEpoB300 are sensitive to albendazole: Involvement of apoptotic pathways. Biochem Pharmacol. 2007;74:407-14. https://doi.org/10.1016/j.bcp.2007.05.006.
Noorani L, Stenzel M, Liang R, Pourgholami MH, Morris DL. Albumin nanoparticles increase the anticancer efficacy of albendazole in ovarian cancer xenograft model. J Nanobiotechnology. 2015;13. https://doi.org/10.1186/s12951-015-0082-8.
Pillai K, Akhter J, Morris DL. Super aqueous solubility of albendazole in β-cyclodextrin for parenteral application in cancer therapy. J Cancer. 2017;8:913-23. https://doi.org/10.7150/jca.17301.
Vinarov Z, Gancheva G, Katev V, Tcholakova SS. Albendazole solution formulation via vesicle-to-micelle transition of phospholipid-surfactant aggregates. Drug Dev Ind Pharm. 2018;44:1130-8. https://doi.org/10.1080/03639045.2018.1438461.
Chassaing C, Berger M, Heckeroth A, Ilg T, Jaeger M, Kern C, et al. Highly water-soluble prodrugs of anthelmintic benzimidazole carbamates: synthesis, pharmacodynamics, and pharmacokinetics. J Med Chem. 2008;51:1111–4. https://doi.org/10.1021/jm701456r.
Strickley RG. Solubilizing excipients in oral and injectable formulations. Pharm Res. 2004;21:201-30. https://doi.org/10.1023/b:pham.0000016235.32639.23.
Yeh MK, Chang LC, Chiou AH. Improving tenoxicam solubility and bioavailability by cosolvent system. AAPS PharmSciTech. 2009;10:166-71. https://doi.org/10.1208/s12249-009-9189-2.
Flores-Ramos M, Ibarra-Velarde F, Hernández-Campos A, Vera-Montenegro Y, Jung-Cook H, Cantó-Alarcón GJ, et al. A highly water soluble benzimidazole derivative useful for the treatment of fasciolosis. Bioorg Med Chem Lett. 2014;24:5814–7. https://doi.org/10.1016/j.bmcl.2014.10.017.
Leahy DK, Pack SK. Preparation of phosphonooxymethyl prodrugs of HIV-1 attachment inhibitors. Org Process Res Dev. 2013;17:1440-44. https://doi.org/10.1021/op400225.
Impurities: Guideline for Residual Solvents Q3C(R8). International council for harmonisation of technical requirements for pharmaceuticals for human use. 2021. https://database.ich.org/sites/default/files/ICH_Q3C-R8_Guideline_Step4_2021_0422_1.pdf Accessed 25 May 2023.
Chistyachenko YS, Khvostov MV, Belousov AI, Zhukova NA, Pakharukova MY, Katokhin AV, et al. Physicochemical properties and anti-opisthorchosis effect of mechanochemically synthesized supramolecular complexes of albendazole with the polysaccharide arabinogalactan from Larix sibirica and Larix gmelinii. Dokl Biol Sci. 2014;456:212–4. https://doi.org/10.1134/S0012496614030156.
Inactive ingredient search for approved drug products. U.S. Food and Drug Administration. 2023. https://www.accessdata.fda.gov/scripts/cder/iig/ Accessed 15 Mar 2023.
Guidance for industry: drug stability guidelines. U.S. Food and Drug Administration. 2008. https://www.fda.gov/media/69957/download Accessed 30 Nov 2022.
Amin K, Dannenfelser RM. In vitro hemolysis: guidance for the pharmaceutical scientist. J Pharm Sci. 2006;95:1173-6. https://doi.org/10.1002/jps.20627.
Zimmermann SC, Tichý T, Vávra J, Dash RP, Slusher CE, Gadiano AJ, et al. N-substituted prodrugs of mebendazole provide improved aqueous solubility and oral bioavailability in mice and dogs. J Med Chem. 2018;61:3918-29. https://doi.org/10.1021/acs.jmedchem.7b01792.
Aleyadeh R, Carson RP. Fosphenytoin. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. 2022. https://www.ncbi.nlm.nih.gov/books/NBK560745/. Accessed 26 March 2023.
Kohri N, Yamayoshi Y, Xin H, Iseki K, Sato N, Todo S, et al. Improving the oral bioavailability of albendazole in rabbits by the solid dispersion technique. J Pharm Pharmacol. 1999;51:159-64. https://doi.org/10.1211/0022357991772277.
Li T, Qiao GL, Hu GZ, Meng FD, Qiu YS, Zhang XY, et al. Comparative plasma and tissue pharmacokinetics and drug residue profiles of different chemotherapeutants in fowls and rabbits. J Vet Pharmacol Ther. 1995;18:260-273. https://doi.org/10.1111/j.1365-2885.1995.tb00590.x.
Alvarez LI, Sánchez SF, Lanusse CE. In vivo and ex vivo uptake of albendazole and its sulphoxide metabolite by cestode parasites: relationship with their kinetic behaviour in sheep. J Vet Pharmacol Ther. 1999;22:77-86. https://doi.org/10.1046/j.1365-2885.1999.00194.x.
Evrard B, Chiap P, DeTullio P, Ghalmi F, Piel G, Van Hees T, et al. Oral bioavailability in sheep of albendazole from a suspension and from a solution containing hydroxypropyl-beta-cyclodextrin. J Control Release. 2002;85:45-50. https://doi.org/10.1016/s0168-3659(02)00270-5.
Dayan AD. Albendazole, mebendazole and praziquantel. Review of non-clinical toxicity and pharmacokinetics. Acta Trop. 2003;86:141-59. https://doi.org/10.1016/s0001-706x(03)00031-7.
Cai ZY, Galettis P, Lu Y, Morris DL, Pourgholami MH. Pharmacokinetics of albendazole in New Zealand white rabbits: oral versus intraperitoneal administration. Anticancer Res. 2007;27:417-22.
Králová V, Hanušová V, Staňková P, Knoppová K, Čáňová K, Skálová L. Antiproliferative effect of benzimidazole anthelmintics albendazole, ricobendazole, and flubendazole in intestinal cancer cell lines. Anticancer Drugs. 2013;24:911-19. https://doi.org/10.1097/CAD.0b013e3283648c69.
Merino G, Jonker JW, Wagenaar E, Pulido MM, Molina AJ, Alvarez AI, et al. Transport of anthelmintic benzimidazole drugs by breast cancer resistance protein (BCRP/ABCG2). Drug Metab Dispos. 2005;33:614-18. https://doi.org/10.1124/dmd.104.003319.
Acknowledgements
We greatly appreciate the support provided by Nayeli Balbiux at USAII UNAM to characterize the prodrug. We are also grateful to Marisol Rivera Huerta and Lucia Macías Rosales for their support in the handling and care of the laboratory animals.
Funding
This work was supported by UNAM-DGAPA-PAPIIT [IN206122]. Student grant (589367) was provided to José Becerril-Vega by CONACYT during the study.
Author information
Authors and Affiliations
Contributions
JBV and HJC conceived/designed the study. JBV, AHC, MFR, and RC contributed to the design and the synthesis of the prodrug. JBV carried out the experiments and data collection. JBV and GLG design the cosolvent formulation. JBV, IGH, and HJC carried out the analysis and interpretation of data. JBV, IGH, LMC, and HJC drafted the manuscript. All authors revised the manuscript critically for intellectual content and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Animal experiments were approved by the Institutional Animal Care and Use Committee (CICUAL) of the Chemistry Faculty, UNAM. Protocol was approved on August 16, 2021, with the official number FQ/CICUAL/435/21. All experiments were conducted according to Mexican Guidance (NOM-062-ZOO-1999).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 5481 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Becerril-Vega, J., Hernández-Campos, A., González-Hernández, I. et al. Development and Pharmacokinetic Evaluation of Two Parenteral Formulations of Albendazole Using Prodrug and Cosolvent Approaches. AAPS PharmSciTech 24, 158 (2023). https://doi.org/10.1208/s12249-023-02613-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02613-9