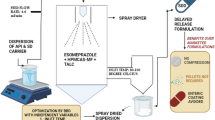
Abstract
The present study adopted a Quality by Design (QbD) approach to spray dry indomethacin nanosuspension (IMC-NS) consisting of HPC-SL, poloxamer 407, and lactose monohydrate. The Box-Behnken Design was used to systematically evaluate the effects of inlet temperature, aspiration rate, and feed rate on the critical quality attributes (CQAs) [redispersibility index (RDI; minimize), % yield (maximize), and % release at 15 min (maximize)] of the indomethacin spray dried nanosuspension (IMC-SD-NS). To identify significant main and quadratic effects, two-way interactions, and create a predictive model for the spray drying process, regression analysis and ANOVA were utilized. Following optimization, the IMC-SD-NS was analyzed for its physicochemical properties using X-ray powder diffraction (XRPD), Fourier transform infrared spectroscopy (FTIR), and in vitro dissolution studies. Statistical analysis revealed significant independent variables, including inlet temperature, feed rate, and aspiration rate, that critically impacted the solidified end product’s RDI, % yield, and % release at 15 min. The models developed for critical quality attributes (CQAs) were significant at a p-value of 0.05. The crystalline state of IMC was maintained in the solidified product, as confirmed by XRPD, and no interactions were observed between IMC and the excipients as evaluated by FTIR. In vitro dissolution studies showed improved dissolution rate for the IMC-SD-NS (3.82-fold increase in overall drug release), which may be attributed to the readily redispersible nanosized drug particles. The implementation of a well-designed study, utilizing Design of Experiments (DoE) methodology, played a crucial role in the development of a highly effective spray drying process.







Similar content being viewed by others
References
Lipinski CA. Drug-like properties and the causes of poor solubility and poor permeability. J Pharm Toxicol Methods. 2000;44(1):235–49.
Tarbit MH, Berman J. High-throughput approaches for evaluating absorption, distribution, metabolism and excretion properties of lead compounds. Current Opin Chem Biol. 1998;2(3):411–6.
Santos A, H, Peltonen L, Limnell T, Hirvonen J. Mesoporous materials and nanocrystals for enhancing the dissolution behavior of poorly water-soluble drugs. Curr Pharm Biotechnol. 2013;14(10):926–38.
Parekh BV, Saddik JS, Patel DB, Dave RH. Evaluating the effect of glidants on tablet sticking propensity of ketoprofen using powder rheology. Int J Pharm. 2023;635:122710.
Dahan A, Miller JM, Amidon GL. Prediction of solubility and permeability class membership: provisional BCS classification of the world’s top oral drugs. The AAPS J. 2009;11:740–6.
Rosenberger J, Butler J, Dressman J. A refined developability classification system. J Pharm Sci. 2018;107(8):2020–32.
Butler JM, Dressman JB. The developability classification system: application of biopharmaceutics concepts to formulation development. J Pharm Sci. 2010;99(12):4940–54.
Savla R, Browne J, Plassat V, Wasan KM, Wasan EK. Review and analysis of FDA approved drugs using lipid-based formulations. Drug Dev Ind Pharm. 2017;43(11):1743–58.
Bennett-Lenane H, O'Shea JP, O'Driscoll CM, Griffin BT. A retrospective biopharmaceutical analysis of> 800 approved oral drug products: are drug properties of solid dispersions and lipid-based formulations distinctive? J Pharm Sci. 2020;109(11):3248–61.
Dugar RP, Gajera BY, Dave RH. Fusion method for solubility and dissolution rate enhancement of ibuprofen using block copolymer poloxamer 407. AAPS PharmSciTech. 2016;17:1428–40.
11: Khatri P, Desai D, Sandhu H, Thongsukmak A, Patel G, Vaghashiya J, Phuapradit W, Shah N. Melt extruded amorphous solid dispersions. In: Pharmaceutical extrusion technology. Boca Raton: CRC Press; 2018. p. 267–310.
Zarmpi P, McAllister M, Butler J, Fotaki N. Biopharmaceutics tools for rational formulation design. Biopharm Fundam Indust Pract. 2022;28:113–33.
Litou C, Patel N, Turner DB, Kostewicz E, Kuentz M, Box KJ, et al. Combining biorelevant in vitro and in silico tools to simulate and better understand the in vivo performance of a nano-sized formulation of aprepitant in the fasted and fed states. Eur J Pharm Sci. 2019;138:105031.
Shringarpure M, Gharat S, Momin M, Omri A. Management of epileptic disorders using nanotechnology-based strategies for nose-to-brain drug delivery. Exp Opin Drug Deliv. 2021;18(2):169–85.
Shah H, Shah K, Gajera B, Dave RH, Taft DR. Developing a formulation strategy coupled with PBPK modeling and simulation for the weakly basic drug albendazole. Pharm. 2023;15(4):1040.
Kvítek L, Panáček A, Soukupova J, Kolář M, Večeřová R, Prucek R, et al. Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles (NPs). The J Phys Chem C. 2008;112(15):5825–34.
Gajera BY, Shah DA, Dave RH. Development of an amorphous nanosuspension by sonoprecipitation-formulation and process optimization using design of experiment methodology. Int J Pharm. 2019;559:348–59.
Gajera BY, Shah DA, Dave RH. Investigating a novel hot melt extrusion-based drying technique to solidify an amorphous nanosuspension using design of experiment methodology. AAPS PharmSciTech. 2018;19(8):3778–90.
Möschwitzer J. Particle size reduction technologies in the pharmaceutical development process. Am Pharm Rev. 2010;2010:54–9.
Rathod V, Stagner WC, Gajera B, Haware RV. Hybridized nanoamorphous micellar dispersion using a QbD–DM3 linked rational product design strategy for ritonavir: A BCS IV drug. Int J Pharm. 2020;588:119727.
Peltonen L, Hirvonen J. Pharmaceutical nanocrystals by nanomilling: critical process parameters, particle fracturing and stabilization methods. J Pharm Pharmacol. 2010;62(11):1569–79.
Liu T, Yu X, Yin H, Möschwitzer JP. Advanced modification of drug nanocrystals by using novel fabrication and downstream approaches for tailor-made drug delivery. Drug Deliv. 2019;26(1):1092–103.
Reverberi AP, Vocciante M, Salerno M, Ferretti M, Fabiano B. Green synthesis of silver nanoparticles by low-energy wet bead milling of metal spheres. Mater. 2019;13(1):63.
Wang Y, Zheng Y, Zhang L, Wang Q, Zhang D. Stability of nanosuspensions in drug delivery. J Controlled Release. 2013;172(3):1126–41.
Van Eerdenbrugh B, Froyen L, Van Humbeeck J, Martens JA, Augustijns P, Van den Mooter G. Drying of crystalline drug nanosuspensions—the importance of surface hydrophobicity on dissolution behavior upon redispersion. Eur J Pharm Sci. 2008;35(1-2):127–35.
Alaei S, Ghasemian E, Vatanara A. Spray drying of cefixime nanosuspension to form stabilized and fast dissolving powder. Powder Technol. 2016;288:241–8.
Malamatari M, Somavarapu S, Taylor KM, Buckton G. Solidification of nanosuspensions for the production of solid oral dosage forms and inhalable dry powders. Exp Opin Drug Deliv. 2016;13(3):435–50.
Beirowski J, Inghelbrecht S, Arien A, Gieseler H. Freeze-drying of nanosuspensions, 1: freezing rate versus formulation design as critical factors to preserve the original particle size distribution. J Pharm Sci. 2011;100(5):1958–68.
Wan KY, Weng J, Wong SN, Kwok PCL, Chow SF, Chow AHL. Converting nanosuspension into inhalable and redispersible nanoparticles by combined in-situ thermal gelation and spray drying. Eur J Pharm Biopharm. 2020;149:238–47.
Patel VD, Rathod V, Haware RV, Stagner WC. Optimized L-SNEDDS and spray-dried S-SNEDDS using a linked QbD-DM3 rational design for model compound ketoprofen. Int J Pharm. 2023;631:122494.
Stagner WC, Iyer M, Rathod V, Meruva S, Staton S, Haware RV. Human volunteer, in vitro, and molecular level evaluation of an optimized taste-masked isoniazid-chitosan spray-dried microparticle matrix. Int J Pharm. 2019;572:118774.
Shah HG, Rathod V, Basim P, Gajera B, Dave RH. Understanding the impact of multi-factorial composition on efficient loading of the stable ketoprofen nanoparticles on orodispersible films using Box-Behnken Design. J Pharm Sci. 2022;111(5):1451–62.
Rathod VR, Shah DA, Dave RH. Systematic implementation of quality-by-design (QbD) to develop NSAID-loaded nanostructured lipid carriers for ocular application: preformulation screening studies and statistical hybrid-design for optimization of variables. Drug Dev Industr Pharm. 2020;46(3):443–55.
Chaturvedi K, Gajera BY, Xu T, Shah H, Dave RH. Influence of processing methods on physico-mechanical properties of Ibuprofen/HPC-SSL formulation. Pharm Dev Technol. 2018;23(10):1108–16.
Tank D, Karan K, Gajera BY, Dave RH. Investigate the effect of solvents on wet granulation of microcrystalline cellulose using hydroxypropyl methylcellulose as a binder and evaluation of rheological and thermal characteristics of granules. Saudi Pharm J. 2018;26(4):593–602.
Gajera BY, Dugar RP, Dave RH. Formulation development and optimization of ibuprofen poloxamer melt granules using hydrophilic excipients. Br J Pharm Res. 2016;13(6):1–19.
Colombo M, Orthmann S, Bellini M, Staufenbiel S, Bodmeier R. Influence of drug brittleness, nanomilling time, and freeze-drying on the crystallinity of poorly water-soluble drugs and its implications for solubility enhancement. Aaps Pharmscitech. 2017;18(7):2437–45.
Patel PJ, Gajera BY, Dave RH. A quality-by-design study to develop Nifedipine nanosuspension: examining the relative impact of formulation variables, wet media milling process parameters and excipient variability on drug product quality attributes. Drug Dev Indust Pharm. 2018;44(12):1942–52.
Yang H, Teng F, Wang P, Tian B, Lin X, Hu X, et al. Investigation of a nanosuspension stabilized by Soluplus® to improve bioavailability. Int J Pharm. 2014;477(1-2):88–95.
Karakucuk A, Teksin ZS, Eroglu H, Celebi N. Evaluation of improved oral bioavailability of ritonavir nanosuspension. Eur J Pharm Sci. 2019;131:153–8.
Rathod V. Optimization and design of ibuprofen-loaded nanostructured lipid carriers using a hybrid-design approach for ocular drug delivery. The Brooklyn Center: Long Island University; 2018.
Dobry DE, Settell DM, Baumann JM, Ray RJ, Graham LJ, Beyerinck RA. A model-based methodology for spray-drying process development. J Pharm Innov. 2009;4:133–42.
Luebbert C, Stoyanov E, Sadowski G. Phase behavior of ASDs based on hydroxypropyl cellulose. Int J Pharm: X. 2021;3:100070.
Lian R, Lu Y, Qi J, Tan Y, Niu M, Guan P, et al. Silymarin glyceryl monooleate/poloxamer 407 liquid crystalline matrices: physical characterization and enhanced oral bioavailability. Aaps Pharmscitech. 2011;12:1234–40.
Tay JBJ, Chua X, Ang C, Subramanian GS, Tan SY, Lin EMJ, et al. Effects of spray-drying inlet temperature on the production of high-quality native rice starch. Process. 2021;9(9):1557.
Dupeyrón D, Kawakami M, Ferreira AM, Cáceres-Vélez PR, Rieumont J, Azevedo RB, et al. Design of indomethacin-loaded nanoparticles: effect of polymer matrix and surfactant. Int J Nanomed. 2013;8:3467.
Karnachi AA, Khan MA. Box-Behnken design for the optimization of formulation variables of indomethacin coprecipitates with polymer mixtures. Int J Pharm. 1996;131(1):9–17.
Li F, Li L, Wang S, Yang Y, Li J, Liu D, et al. Improved dissolution and oral absorption by co-grinding active drug probucol and ternary stabilizers mixtures with planetary beads-milling method. Asian J Pharm Sci. 2019;14(6):649–57.
Sigfridsson K, Lundqvist AJ, Strimfors M. Particle size reduction for improvement of oral absorption of the poorly soluble drug UG558 in rats during early development. Drug Dev Indust Pharm. 2009;35(12):1479–86.
Otsuka Y. Chemometrics evaluation of phase transformations based on ATR-IR spectra and powder X-ray diffractogram (Doctoral dissertation, 徳島大学). 2018.
Housheh S, Trefi S, Chehna MF. Identification and characterization of prasugrel degradation products by GC/MS, FTIR and 1H NMR. Asian J Pharm Anal. 2017;7(2):55–66.
Mokarram AR. Preparation and in-vitro evaluation of indomethacin nanoparticles. DARU J Pharm Sci. 2010;18(3):185.
Acknowledgements
The authors are thankful to Division of Pharmaceutical Sciences, Long Island University to provide an opportunity to conduct the above research.
Author information
Authors and Affiliations
Contributions
Vishal Rathod: methodology, investigation, writing – original draft, experimentation, visualization, validation, investigation, software.
Anusha Pinninti: experimentation, methodology, formal analysis, data curation.
Bhavin Gajera: methodology, supervision, writing - review & editing.
Irfan A. Mohammed: formal analysis, writing - review & editing.
Rutesh Dave: conceptualization, supervision, writing - review & editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 15 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rathod, V., Gajera, B., Pinninti, A. et al. Strategizing Spray Drying Process Optimization for the Manufacture of Redispersible Indomethacin Nanoparticles Using Quality-by-Design Principles. AAPS PharmSciTech 24, 133 (2023). https://doi.org/10.1208/s12249-023-02589-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02589-6