Abstract
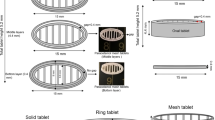
Apnea of prematurity can be treated with a body-weight-adjusted dosage of caffeine. Semi-solid extrusion (SSE) 3D printing represents an interesting approach to finely tailor personalized doses of active ingredients. To improve compliance and ensure the right dose in infants, drug delivery systems such as oral solid forms (orodispersible film, dispersive form, and mucoadhesive form) can be considered. The aim of this work was to obtain a flexible-dose system of caffeine by SSE 3D printing by testing different excipients and printing parameters. Gelling agents (sodium alginate (SA) and hydroxypropylmethyl cellulose (HPMC)) were used to obtain a drug-loaded hydrogel matrix. Disintegrants (sodium croscarmellose (SC) and crospovidone (CP)) were tested for get rapid release of caffeine. The 3D models were patterned by computer-aided design with variable thickness, diameter, infill densities, and infill patterns. The oral forms produced from the formulation containing 35% caffeine, 8.2% SA, 4.8% HPMC, and 52% SC (w/w) were found to have good printability, achieving doses approaching to those used in neonatology (between 3 and 10 mg of caffeine for infants weighing approximately between 1 and 4 kg). However, disintegrants, especially SC, acted more as binder/filler, showing interesting properties to maintain the shape after extrusion and enhance printability without a significant effect on caffeine release.
Graphical Abstract






Similar content being viewed by others
References
Kochanowska-Karamyan AJ. Pharmaceutical compounding: the oldest, most symbolic, and still vital part of pharmacy. Int J Pharm Compd. 2016;20(5):367–74.
Brion F, Nunn AJ, Rieutord A. Extemporaneous (magistral) preparation of oral medicines for children in European hospitals. Acta Paediatr. 2003;92(4):486–90.
Rahman Z, Barakh Ali SF, Ozkan T, Charoo NA, Reddy IK, Khan MA. Additive manufacturing with 3D printing: progress from bench to bedside. AAPS J. 2018;20(6):101.
Annereau M, Toussaint B, Dufaÿ Wojcicki A, Dufaÿ S, Diaz Salmeron R, Boudy V. Impression 2D–3D dans les pharmacies hospitalières: quels rôles et quels challenges ? Ann Pharm Fr. 2021;79(4):361–74.
ISO/ASTM 52900:2021(en), Additive manufacturing — general principles — Fundamentals and vocabulary [Internet]. 2021. Available from: https://www.iso.org/obp/ui/#iso:std:iso-astm:52900:ed-2:v1:en. Accessed 5 Aug 2022.
Wang S, Chen X, Han X, Hong X, Li X, Zhang H, et al. A Review of 3D Printing Technology in Pharmaceutics: Technology and Applications, Now and Future. Pharmaceutics. 2023;15(2):416.
Herrada-Manchón H, Rodríguez-González D, Alejandro Fernández M, Suñé-Pou M, Pérez-Lozano P, García-Montoya E, et al. 3D printed gummies: personalized drug dosage in a safe and appealing way. Int J Pharm. 2020;587: 119687.
Seoane-Viaño I, Januskaite P, Alvarez-Lorenzo C, Basit AW, Goyanes A. Semi-solid extrusion 3D printing in drug delivery and biomedicine: personalised solutions for healthcare challenges. J Control Release. 2021;332:367–89.
Scoutaris N, Ross SA, Douroumis D. 3D Printed ‘starmix’ drug loaded dosage forms for paediatric applications. Pharm Res. 2018;35(2):34.
Wang Z, Han X, Chen R, Li J, Gao J, Zhang H, et al. Innovative color jet 3D printing of levetiracetam personalized paediatric preparations. Asian J Pharm Sci. 2021;16(3):374–86.
Patel SK, Khoder M, Peak M, Alhnan MA. Controlling drug release with additive manufacturing-based solutions. Adv Drug Deliv Rev. 2021;174:369–86.
Govender R, Kissi EO, Larsson A, Tho I. Polymers in pharmaceutical additive manufacturing: a balancing act between printability and product performance. Adv Drug Deliv Rev. 2021;177: 113923.
Krause J, Müller L, Sarwinska D, Seidlitz A, Sznitowska M, Weitschies W. 3D Printing of mini tablets for pediatric use. Pharmaceuticals (Basel). 2021;14(2):143.
Palekar S, Nukala PK, Mishra SM, Kipping T, Patel K. Application of 3D printing technology and quality by design approach for development of age-appropriate pediatric formulation of baclofen. Int J Pharm. 2019;556:106–16.
Goyanes A, Madla CM, Umerji A, Duran Piñeiro G, Giraldez Montero JM, Lamas Diaz MJ, et al. Automated therapy preparation of isoleucine formulations using 3D printing for the treatment of MSUD: first single-centre, prospective, crossover study in patients. Int J Pharm. 2019;567: 118497.
Khaled SA, Burley JC, Alexander MR, Yang J, Roberts CJ. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J Control Release. 2015;217:308–14.
Khaled SA, Burley JC, Alexander MR, Yang J, Roberts CJ. 3D printing of tablets containing multiple drugs with defined release profiles. Int J Pharm. 2015;494(2):643–50.
Rahman J, Quodbach J. Versatility on demand – the case for semi-solid micro-extrusion in pharmaceutics. Adv Drug Deliv Rev. 2021;172:104–26.
Abdella S, Youssef SH, Afinjuomo F, Song Y, Fouladian P, Upton R, et al. 3D printing of thermo-sensitive drugs. Pharmaceutics. 2021;13(9):1524.
Auriemma G, Tommasino C, Falcone G, Esposito T, Sardo C, Aquino RP. Additive manufacturing strategies for personalized drug delivery systems and medical devices: fused filament fabrication and semi solid extrusion. Molecules. 2022;27(9):2784.
Rodríguez-Pombo L, Awad A, Basit AW, Alvarez-Lorenzo C, Goyanes A. Innovations in chewable formulations: the novelty and applications of 3D printing in drug product design. Pharmaceutics. 2022;14(8):1732.
Januskaite P, Xu X, Ranmal SR, Gaisford S, Basit AW, Tuleu C, et al. I Spy with my little eye: a paediatric visual preferences survey of 3D printed tablets. Pharmaceutics. 2020;12(11):1100.
Karavasili C, Gkaragkounis A, Moschakis T, Ritzoulis C, Fatouros DG. Pediatric-friendly chocolate-based dosage forms for the oral administration of both hydrophilic and lipophilic drugs fabricated with extrusion-based 3D printing. Eur J Pharm Sci. 2020;147: 105291.
Zhu C, Tian Y, Zhang E, Gao X, Zhang H, Liu N, et al. Semisolid extrusion 3D printing of propranolol hydrochloride gummy chewable tablets: an innovative approach to prepare personalized medicine for pediatrics. AAPS PharmSciTech. 2022;23(5):166.
Taketomo CK, Hodding JH, Pediatric & neonatal dosage handbook, 29th Ed., ACCP, 2022.
Smith J, Marks C. In-use microbiological assessment of caffeine citrate 10 mg/mL oral solution. Eur J Hosp Pharm. 2018;25(e2):e130–3.
Castro PM, Sousa F, Magalhães R, Ruiz-Henestrosa VMP, Pilosof AMR, Madureira AR, et al. Incorporation of beads into oral films for buccal and oral delivery of bioactive molecules. Carbohydr Polym. 2018;194:411–21.
Germini G, Peltonen L. 3D printing of drug nanocrystals for film formulations. Molecules. 2021;26(13):3941.
Cui M, Li Y, Wang S, Chai Y, Lou J, Chen F, et al. Exploration and preparation of a dose-flexible regulation system for levetiracetam tablets via novel semi-solid extrusion three-dimensional printing. J Pharm Sci. 2019;108(2):977–86.
Yan TT, Lv ZF, Tian P, Lin MM, Lin W, Huang SY, et al. Semi-solid extrusion 3D printing ODFs: an individual drug delivery system for small scale pharmacy. Drug Dev Ind Pharm. 2020;46(4):531–8.
Eduardo DT, Ana SE, José BF. A micro-extrusion 3D printing platform for fabrication of orodispersible printlets for pediatric use. Int J Pharm. 2021;605: 120854.
Pamlényi K, Kristó K, Jójárt-Laczkovich O, Regdon G. Formulation and optimization of sodium alginate polymer film as a buccal mucoadhesive drug delivery system containing cetirizine dihydrochloride. Pharmaceutics. 2021;13(5):619.
Azad MA, Olawuni D, Kimbell G, Badruddoza AZM, Hossain MdS, Sultana T. Polymers for extrusion-based 3D printing of pharmaceuticals: a holistic materials–process perspective. Pharmaceutics. 2020;12(2):124.
C Rowe R, J Sheskey P, E Quinn M. Handbook of pharmaceutical excipients. Sixth edition. Pharmaceutical Press and American Pharmacists Association; 2009.
Bom S, Ribeiro R, Ribeiro HM, Santos C, Marto J. On the progress of hydrogel-based 3D printing: correlating rheological properties with printing behaviour. Int J Pharm. 2022;615: 121506.
European Directorate for the Quality of Medicines & Healthcare, Council of Europe. 2.9.5. Uniformité de masse des... - European pharmacopoeia 10.6 [Internet]. [cited 2022 Mar 1]. Available from: https://pheur.edqm.eu/app/10-6/content/10-6/20905F.htm?highlight=on&terms=uniformit%C3%A9&terms=uniformit%C3%A9%20de%20masse&terms=de&terms=masse&terms=de%20masse&terms=d%E2%80%99uniformit%C3%A9%20de%20masse&terms=d%E2%80%99uniformit%C3%A9%20de. Accessed 1 Mar 2022.
European Directorate for the Quality of Medicines & Healthcare, Council of Europe. 2.9.1. Disintegration of tablets and capsules - European pharmacopoeia 10.7 [Internet]. Available from: https://pheur.edqm.eu/app/10-7/content/10-7/20901E.htm?highlight=on&terms=disintegration. Accessed 10 Aug 2022.
European Directorate for the Quality of Medicines & Healthcare, Council of Europe. 2.9.3. Dissolution test for solid dosage forms - European pharmacopoeia 10.7 [Internet]. Available from: https://pheur.edqm.eu/app/10-7/content/10-7/20903E.htm. Accessed 10 Aug 2022.
Hölzl K, Lin S, Tytgat L, Van Vlierberghe S, Gu L, Ovsianikov A. Bioink properties before, during and after 3D bioprinting. Biofabrication. 2016;8(3): 032002.
Gholamipour-Shirazi A, Norton IT, Mills T. Designing hydrocolloid based food-ink formulations for extrusion 3D printing. Food Hydrocolloids. 2019;95:161–7.
Axpe E, Oyen ML. Applications of alginate-based bioinks in 3D bioprinting. Int J Mol Sci. 2016;17(12):1976.
Dai L, Cheng T, Duan C, Zhao W, Zhang W, Zou X, et al. 3D printing using plant-derived cellulose and its derivatives: a review. Carbohydr Polym. 2019;203:71–86.
Panraksa P, Qi S, Udomsom S, Tipduangta P, Rachtanapun P, Jantanasakulwong K, et al. Characterization of hydrophilic polymers as a syringe extrusion 3D printing material for orodispersible film. Polymers (Basel). 2021;13(20):3454.
Hölzl K, Lin S, Tytgat L, Van Vlierberghe S, Gu L, Ovsianikov A. Bioink properties before, during and after 3D bioprinting. Biofabrication. 2016;8(3): 032002.
Wang J, Liu Y, Zhang X, Rahman SE, Su S, Wei J, et al. 3D printed agar/ calcium alginate hydrogels with high shape fidelity and tailorable mechanical properties. Polymer. 2021;214: 123238.
Bom S, Santos C, Barros R, Martins AM, Paradiso P, Cláudio R, et al. Effects of starch incorporation on the physicochemical properties and release kinetics of alginate-based 3D hydrogel patches for topical delivery. Pharmaceutics. 2020;12(8):719.
Kim MH, Lee YW, Jung WK, Oh J, Nam SY. Enhanced rheological behaviors of alginate hydrogels with carrageenan for extrusion-based bioprinting. J Mech Behav Biomed Mater. 2019;98:187–94.
Gao T, Gillispie GJ, Copus JS, Pr AK, Seol YJ, Atala A, et al. Optimization of gelatin-alginate composite bioink printability using rheological parameters: a systematic approach. Biofabrication. 2018;10(3): 034106.
Cerciello A, Del Gaudio P, Granata V, Sala M, Aquino RP, Russo P. Synergistic effect of divalent cations in improving technological properties of cross-linked alginate beads. Int J Biol Macromol. 2017;101:100–6.
Suárez-González J, Magariños-Triviño M, Díaz-Torres E, Cáceres-Pérez AR, Santoveña-Estévez A, Fariña JB. Individualized orodispersible pediatric dosage forms obtained by molding and semi-solid extrusion by 3D printing: a comparative study for hydrochlorothiazide. J Drug Deliv Sci Technol. 2021;66: 102884.
Hu J, Fitaihi R, Abukhamees S, Abdelhakim HE. Formulation and characterisation of carbamazepine orodispersible 3D-printed mini-tablets for paediatric use. Pharmaceutics. 2023;15(1):250.
Tagami T, Ando M, Nagata N, Goto E, Yoshimura N, Takeuchi T, et al. Fabrication of naftopidil-loaded tablets using a semisolid extrusion-type 3D printer and the characteristics of the printed hydrogel and resulting tablets. J Pharm Sci. 2019;108(2):907–13.
Berardi A, Bisharat L, Quodbach J, Abdel Rahim S, Perinelli DR, Cespi M. Advancing the understanding of the tablet disintegration phenomenon – an update on recent studies. Int J Pharm. 2021;598: 120390.
Liu Y, Weng R, Wang W, Wei X, Li J, Chen X, et al. Tunable physical and mechanical properties of gelatin hydrogel after transglutaminase crosslinking on two gelatin types. Int J Biol Macromol. 2020;162:405–13.
Sarode AL, Sandhu H, Shah N, Malick W, Zia H. Hot melt extrusion for amorphous solid dispersions: temperature and moisture activated drug-polymer interactions for enhanced stability. Mol Pharm. 2013;10(10):3665–75.
Suntornnond R, Tan EYS, An J, Chua CK. A mathematical model on the resolution of extrusion bioprinting for the development of new bioinks. Materials (Basel). 2016;9(9):E756.
European Directorate for the Quality of Medicines & Healthcare, Council of Europe. Tablets - European pharmacopoeia 10.7 [Internet]. Available from: https://pheur.edqm.eu/app/10-7/content/10-7/0478E.htm?highlight=on&terms=tablet&terms=tablet&terms=tablet&terms=orodispersible&terms=orodispersible&terms=orodispersible&terms=orodispersible&terms=tablet&terms=tablet. Accessed 11 Aug 2022.
Author information
Authors and Affiliations
Contributions
A.R., N.M.S.-B., and I.S.: conceptualization. A.R. and N.M.S.-B.: data curation. A.R., N.M.S.-B., A.A., and J.-C.R.: investigation. A.R., N.M.S.-B., S.B., and I.S.: methodology. N.M.S.-B. and A.A.: software. I.S.: supervision. N.M.S.-B. and I.S.: validation. N.M.S.-B. and I.S.: visualization. A.R.: writing—original draft. N.M.S.-B. and I.S: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Roche, A., Sanchez-Ballester, N.M., Aubert, A. et al. Preliminary Study on the Development of Caffeine Oral Solid Form 3D Printed by Semi-Solid Extrusion for Application in Neonates. AAPS PharmSciTech 24, 122 (2023). https://doi.org/10.1208/s12249-023-02582-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02582-z