Abstract
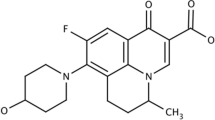
Mixed polymeric micelles are potential nanocarriers for topical drug delivery. Dapsone (DAP) is an antibacterial used as anti-acne agent, but challenged by low water solubility and poor skin permeability. In the present study, DAP-loaded mixed micellar gel was developed comprising Pluronics F-68 and F-127. Micelles were prepared by solvent evaporation method and particle size, ex vivo permeation, drug loading, and entrapment efficiency were determined. Central Composite Design was used to optimize formulation. Independent variables were concentration of Pluronics at three levels while micelle size and drug loading capacities were dependent variables. Droplet size ranged from 400 to 500 nm. Transmission electron microscopy revealed spherical morphology of micelles. Optimized micelles were incorporated into gel base using HPMC K100M, Sodium CMC, and Carbopol 980 as gelling agents. Gels were evaluated for pH, drug content, spreadability, rheology, syneresis, ex vivo permeation, and subacute dermal toxicity. Compared with solubility of free DAP (0.24+0.056 µg/ml), solubility in mixed micelles was 18.42±3.4 µg/ml in water at room temperature. Order of spreadability of gels was Na CMC < HPMC < Carbopol 980. Carbopol gels displayed thixotropy with index of 3.17. Syneresis for all gels from day 0 to day 30 was found to be in range of 4.2 to 15.6% w/w. Subacute dermal toxicity studies showed no signs of erythema and edema on rat skin until 21 days. These results suggest that mixed micelles can significantly increase solubility and permeability and sustain release of DAP and are suitable carriers for topical DAP delivery in anti-acne therapies.
Graphical Abstract







Similar content being viewed by others
Data Availability
Data will be made available if required.
References
Ghaoui N, Hanna E, Abbas O, Kibbi AG, Kurban M. Update on the use of dapsone in dermatology. Int J Dermatol. 2020Jul;59(7):787–95.
Lynn D, Umari T, Dunnick C. The epidemiology of acne vulgaris in late adolescence. Adolesc Health Med Ther. 2016;7:13–25.
Skroza N, Tolino E, MambrinS A, Zuber C, Marchesiello A, Bernardini C. Adult acne versus adolescent acne: a retrospective study of 1,167 patients. J Clin AesthetDermatol. 2018; 21-25.
McLaughlin J, Watterson S, Layton A, Bjourson A, Barnard E, McDowell A. Microorganisms. 2019;7(5):128.
Suppiah T, Sundram, T, Tan E, Lee C. Bustami N, Tan K. Acne vulgaris and its association with dietary intake: a Malaysian perspective. Asia Pac J Clin Nutr. 2018; 275: 11141-5.
Irby C, Yentzer B, Feldman S. A review of adapalene in the treatment of acne vulgaris. Adolesc J Health. 2008;43:421–4.
Parra A, Jarak I, Santos A, Veiga F, Figueiras A. Micelles polymeric: a promising pathway for dermal drug delivery. Materials. 2021;14:7278.
Hazarika N, Archana M. The psychosocial impact of acne vulgaris. Indian J Dermatol. 2016;61(5):515–20.
Misery L. Consequences of psychological distress in adolescents with acne. J Invest Dermatol. 2011;131:290–2.
Wozel G, Blasum C. Dapsone in dermatology and beyond. Arch Dermatol Res. 2014;306(2):103–24.
Radley K, Tucker R. DAP in the management of Acne vulgaris. Derma J Nurses’ Asso. 2013;6:316–9.
Habib R, Abdeltawab and Ibtehals N. D-optimal mixture design for optimization of topical DAPniosomes: in vitro characterization and in vivo activity against Cutibacterium acne. Drug Del. 2022;29: 821-36.
Nickles M and E. Lake. Topical DAP in treatment of acne: a systemic review. Int J Dermatol. 2022;61(11):1412-21.
Vinicius R, Alice S, Adrian R, Lucio M, Valeria P. Nanoemulsion containing DAP for topical administration: a study of in vitro release and epidermal permeation. Int J Nanomed. 2013;8:535–44.
Meraj A, Kanoujia P, Parashar M, Arya A, Yadav and Saraf S. Evaluation of a Polymer-Lipid-Polymer system utilizing hybrid nanoparticles of DAP as a novel antiacne Agent. Curr Drug Ther. 2016;11(2):86-10.
Mahore J, Suryavanshi S, Shirolkar S, Deshkar S. Enhancement of percutaneous delivery of DAP by microemulsion gel. J Young Pharm. 2017;9(4):507–12.
Rodrigo C, Marchi J, Bergamo V, Fuentefria A, Lavayen V, Guterres S, Pohlmann A, Chitosan-coated dapsone-loaded lipid-core nanocapsules: growth inhibition of clinical isolates, multidrug-resistant Staphylococcus aureus and Aspergillus sp., Coll and Surf A: Physicochem and Engg Aspects. 2016; 511, 153-161.
Mehdi R, Payam K, Pardakhtya A, Tahamipour B, Amanatfar A. Preparation of polyacrylamide/polylactic acid co-assembled core/shell nanofibers as designed beads for dapsone in vitro efficient delivery Artificial Cells. Nanomedicine, And Biotech. 2019;47(1):917–26.
Luis R, Fernandez M, Deb S, Molly M. Stevens, Julio San Roman, Designing dapsone polymer conjugates for controlled drug delivery. Acta Biomater. 2015;27:32–41.
Hemant B, Leena K, KartikN, Vinay V, Kalyani S. Phytoconstituent plumbagin: chemical, biotechnological and pharmaceutical aspects. Studies in Natural Products Chemistry.2019; 63: 415-60.
Tharwat F. Surfactants, Industrial Applications, Robert A. Meyers. Encyclopedia of Physical Science and Technology (Third Edition), Academic Press.2003,423-38.
Chandramani P, Vaidya U, Shashibhal P in Mechanism for development of nanobased drug delivery system, applications of targeted nano drugs and delivery systems ,Nanosci. Nanotech. Drug Del, Micro and Nano Technologies. 2019:35-67.
Lapteva M, Mondon K, Möller M, Gurny R, Kalia Y. Polymeric micelle nanocarriers for the cutaneous delivery of tacrolimus: a targeted approach for the treatment of psoriasis. Mol Pharm. 2014;11:2989–3001.
Makhmalzade B, Chavoshy F. Polymeric micelles as cutaneous drug delivery system in normal skin and dermatological disorders. J Adv Pharm Technol Res. 2018;9(1):2–8.
Yang J, Park H, Koo N, Shin T, Lee E, Cho S. Development of polymeric micelles of oleanolic acid and evaluation of their clinical efficacy. Nanoscale Res Lett. 2020;15:1–14.
Biniek K, Kaczvinsky J, Matts P, Dauskardt R. Understanding age-induced alterations to the biomechanical barrier function of human stratum corneum. J Dermatol Sci. 2015;80:94–101.
Kandekar S, del Río-Sancho S, Lapteva M, Kalia Y. Selective delivery of adapalene to the human hair follicle under finite dose conditions using polymeric micelle nanocarriers. Nanoscale. 2018;10:1099–110.
Chavoshy F, Zadeh B, TamaddonA, Anbardar M. Delivery and anti-psoriatic effect of silibinin-loaded polymeric micelles: an experimental study in the psoriatic skin model. Curr Drug Deliv. 2020;17:787-98.
Shawesh A, Kallioinen S, Hellén L, Antikainen O, Yliruusi J. Pluronic F-127 gels as a vehicle for topical formulations of indomethacin and rheological behaviour of these formulations. Pharmazie. 2002;57(3):186–90.
Alvarado-Gomez E, Martínez-Castañon G, Sanchez R, Ganem-Rondero A, Jose M, Martinez-Gutierrez F. Evaluation of anti-biofilm and cytotoxic effect of a gel formulation with Pluronic F-127 and silver nanoparticles as a potential treatment for skin wounds. MatrLSci and Engg: C. 2018;92:621–30.
Xu J, Wei Z, Jie S, Jia H, Haisheng Q, Xulin C, Xianwen W. Thermosensitive hydrogel loaded with nickel–copper bimetallic hollow nanospheres with SOD and CAT enzymatic-like activity promotes acute wound healing. ACS Appl Mater Interfaces. 2022;14(45):50677–91.
Wang C, Wu B, Wu Y, Song X, Zhang S, Liu Z. Camouflaging nanoparticles with brain metastatic tumor cell membranes: a new strategy to traverse blood–brain barrier for imaging and therapy of brain tumors. AdvFunct Mater. 2020;30:1909369.
Yu J, Qiu H, Yin S, Wang H, Li Y. Polymeric drug delivery system based on pluronics for cancer treatment. Molecules. 2021;26(12):3610.
Trujillo M, Schramm M. Measuring critical micelle concentration as a function of cavity and additives using surface tension and dye micellization. Ronald E McNair Postbac Achiev Program. 2010;14:155–68.
Rao M, Godbole R, Borate S, Mahajan S, Gangwal T. Nanosuspension coated multiparticulates for controlled delivery of albendazole. Drug Dev Ind Pharm. 2021;47(3):367–76.
Ashjari M, Khoee S, Mahdavian A, Rahmatolahzadeh R. Self-assembled nanomicelles using PLGA-PEG amphiphilic block copolymer for insulin delivery: a physicochemical investigation and determination of CMC values. J Matr Sci Matr Med. 2012;23(4):943–53.
Jaiswal M, Kumar M, Pathak K. Zero order delivery of itraconazole via polymeric micelles incorporated in situ ocular gel for the management of fungal keratitis. Coll Surf B Biointer. 2015;1(30):23–30.
Qumbar M, Ameedu Z, Imam S, Javed A, Ahmad J, Ali A. Formulation and optimization of Lacidipine loaded niosomal gel for transdermal delivery: In vitro characterization and In vivo activity. Biomed Pharmacother. 2017;93:255–66.
https://wiki.anton-paar.com/in-en/basics-of-thixotropy. Accessed on 10.08.2022.
Borman P, Elder D. Q2 (R1) Validation of analytical procedures. ICH Quality guidelines. 2017;5:127–66.
Abd E, Shereen A, Pastore M, Telaprolu K, Mohammed Y, Namjoshi S, Grice J, Roberts M. Skin models for the testing of transdermal drugs clinical pharmacology: advances and Applications. 2016;8:163–76.
OECD (1981) Test no. 410: repeated dose dermal toxicity: 21/28- day study, OECD Guidelines for the testing of Chemicals, Section 4. OECD Publishing, Paris. https://doi.org/10.1787/9789264070 745-en.
Samhitha K, Dmitry B, Marina T, Paschalis A. Structure and composition of mixed micelles formed by nonionic block copolymers and ionic surfactants in water determined by small-angle neutron scattering with contrast variation. J Coll and Inter Sci. 2022;609:456–68.
Pedersen J, Gerstenberg M. The structure of P85 Pluronic block copolymer micelles determined by small-angle neutron scattering, Collo.Surf., A 213 (2-3) (2003) 175–187.
Li X, Zhang Y, Fan Y, Zhou Y, Wang X, Fan C, Liu Y, Zhang Q. Preparation and evaluation of novel mixed micelles as nanocarriers for intravenous delivery of propofol. Nanoscale Res Lett. 2011;6:275.
Milton J, Rosen, Surfactants and interfacial phenomena. Third edition, A John Wiley & Sons, Inc.,John Wiley & Sons, Inc., 2004, 415-427.
Feng J, Wu S, Wang H, Liu S. Stability of trianionic curcumin enhanced by gemini alkyl O-Glucosides and alkyl trimethyl ammonium halides mixed micelles. Colloids Surf A Physicochem Eng Asp. 2016;504:190–200.
Carlota O, Adalberto P, Leoberta C. Micellar solubilization of drugs. J Pharm Pharmaceu Sci. 2005;8(2):147–63.
Tijana R, Kasagić I, Jovanović M, Stojanović B, Ivanović D. Comparison of full factorial design, central composite design, and Box-Behnken design in chromatographic method development for the determination of fluconazole and its impurities. Anal Lett. 2014;47(8):1334–7.
Oliver R, Lipfert J, Fox D, Lo R, Doniach S, Columbus L. Dependence of micelle size and shape on detergent alkyl chain length and head group. PLoS One. 2013;8(5):E62488.
Dupuy C, Auvray X, Petipas C, Anthore R, Costes F, Rico-Lattes I, Lattes A. Small angle X-ray and neutron scattering study of the micellization of (N-Alkylamino)-1-deoxylactitols in water. Langm. 1996;12:3162–72.
Charles T. Micelle shape and size. J Phys Chem. 1972;76(21):3020.
Mortensen K. Structural properties of self-assembled polymeric micelles. CurrOpi Coll Interf Sci. 1998;3:12–9.
Rajak P, Nath L, Bhuyan B. Liquid crystals: an approach in drug delivery. Ind J Pharm Sci. 2019;81(1):11–21.
Alexandridis P, Zhou D, Khan A. Lyotropic liquid crystallinity in amphiphilic block copolymers: temperature effects on phase behavior and structure for poly(ethylene oxide)- b-poly(propylene oxide)-b-poly(ethylene oxide) triblock copolymers of different composition. Lang. 1996;12:2690–700.
Danaei M, Dehghankhold M, Ataei S, Hasanzadeh D, Javanmard R, Dokhani A, Khorasani S, Mozafari M. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10:57.
Bence S, Csóka I, Budai M, Kozma G, Berkesi D, Kónya Z, György B, Katona G. Development of dexamethasone-loaded mixed polymeric micelles for nasal delivery. Eur J Pharm Sci. 2021;166:105960.
Kataoka K, Harada A. Yukio Nagasaki, Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv Drug Del Rev. 2012;64:37–48.
Lu Y, Park K. Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int J Pharm. 2013;453:198–214.
Sushant K, Yogesh C, Nazma I, Vishnukant M. Polymeric micelles: authoritative aspects for drug delivery. Des Monomers Polym. 2012;15(5):465–521.
Zarafshani Z, Akdemir O, Lutz J. A ‘“click”’ strategy for tuning in situ the hydrophilic- hydrophobic balance of AB macrosurfactants. Macromol Rapid Comm. 2008;29:1161–6.
Hongbo F, Xinyi L, Weiyu W, Nam-Goo K, Jimmy W. Block copolymers: synthesis, self-assembly, and applications. Polymers. 2017;9:494.
Forster S, Plantenberg T. From self-organizing polymers to nanohybrid and biomaterials. Angewandte Chemie International Edition. 2002;41:688–714.
https://www.sigmaaldrich.com/IN/en/product/sigma/p1300?gclid=Cj0KCQjw54iXBhCXARIsADWpsG9sCOtkPORIdSHREVKnq756sj7LZc2uzl_yxASiYBXR3qhU4jC02UkaAiWuEALw_wcB) [Accessed on 3.08.2022].
https://www.sigmaaldrich.com/IN/en/product/sigma/p2443?gclid=Cj0KCQjw54iXBhCXARIsADWpsG-01oteQfqulJ_7mUk80wgoPGxL_i7AJelCCkDaUYhMsQ9QyadAI0aAuHxEALw_wcB [ Accessed on 3.08.2022].
Sinko P, Martin A. Measurement of thixotropy in Martin's Physical Pharmacy and Pharmaceutical Sciences, 5th ed., Lippincott Williams & Wilkins, 2005,567.
Chi L, Venkat M, Yugyung L. Thixotropic property in pharmaceutical formulations. J Contr Rel. 2009;136:88–98.
https://www.corrosionpedia.com/definition/2526/thixotropic-index-ti [Accessed on 10.08.2022].
A.S. Lubansky in Chapter: Medical Biotechnology and Healthcare, in Comprehensive Biotechnology, 2nd edition, Volume 5. 2011, ed: Murray Moo Young, Elsevier Publications, Boston USA, 189-201.
Antonio F, Iván J, Sierra B, Fernández A, Javier F, Manuel M, Rubio J, Enrique L. Gels andmicrogels for nanotechnological applications. Adv Coll Interf Sci. 2009;147–148:88–108.
Zhao Z, Wang Q, Zhang L, Wu T. Structured water and water-polymer interactions in hydrogels of molecularly imprinted polymers. J Phys Chem B. 2008;112(25):7515–21.
Björn L, Gunnar K, Lars S. On the mechanism of dissolution of cellulose. J Mol Liq. 2010;156(1):76–81.
Ali K, Sylvain R. Interaction effects between cellulose and water in nanocrystalline and amorphous regions: a novel approach using molecular modeling. J Nanomat. 2013, Article ID 409676.
Conti S, Maggi L, Segale L, Ochoa Machiste E, Conte U, Grenier P, Vergnault G. Matrices containing NaCMC and HPMC 2. Swelling and release mechanism study. Int J Pharm. 2007, 21;333(1-2):143-51.
Safitri F, Nawangsari D, Febrina D. Overview: application of Carbopol 940 in Gel. Advances in Health Sciences Research, Volume 34 Proceedings of the International Conference on Health and Medical Sciences (AHMS 2020), 80-84.
Zheng Y, Ouyang W, Wei Y, Syed S, Hao C, Wang B, Shang Y. Effects of Carbopol® 934 proportion on nanoemulsion gel for topical and transdermal drug delivery: a skin permeation study. Int J Nanomedicine. 2016;11:5971–87.
Shegaokar, R. Souto, E. ‘Skin penetration of nanoparticles’ in emerging nanotechnologies in immunology; Eds. Nafisi S, Maibach H, 2018, Elsevier Publications, Boston, MA, USA, 47–88.
Güngör S, Kahraman E. Nanocarriers mediated cutaneous drug delivery. Eur J Pharm Sci. 2021;158:105638.
Dahmana N, Mugnier T, Gabriel D, Favez T, Kowalczuk L, Behar-Cohen F, Gurny R, Kalia YN. Polymeric micelle mediated follicular delivery of spironolactone: targeting the mineralocorticoid receptor to prevent glucocorticoid-induced activation and delayed cutaneous wound healing. Int J Pharm. 2021;604:120773.
Kahraman E, Ÿzhan G, Ÿzsoy Y, Güngör S. Polymeric micellar nanocarriers of benzoyl peroxide as potential follicular targeting approach for acne treatment. Coll Surf B Bioin. 2016;146:692–9.
Acknowledgements
We thank the Department of Science and Technology (DST), Ministry of Science and Technology, Government of India, for their support for TEM analysis of sample. The authors would also like to thank Dr. Makarand Kulkarni, Scientific Expert, Instrumentation Center, Punyashlok Ahilyadevi Holkar Solapur University, Solapur, for the residual solvent analysis by GC-MS.
Funding
This work is self-funded and it has not received funding from any government or nongovernment sources.
Author information
Authors and Affiliations
Contributions
Dr. Monica RP Rao: conceptualization; data curation, writing—review and editing.
Mr. Sushant Deshpande: formal analysis, investigation; methodology; resources; software
Dr. Padmanabh Deshpande: supervision; validation; visualization
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rao, M.R., Deshpande, S. & Deshpande, P. Dapsone-Loaded Mixed Micellar Gel for Treatment OF Acne Vulgaris. AAPS PharmSciTech 24, 109 (2023). https://doi.org/10.1208/s12249-023-02564-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02564-1