Abstract
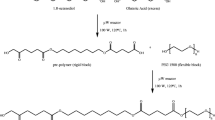
The present study was undertaken to synthesize PEGylated monomethoxy poly (ethylene glycol)-poly (ε-Caprolactone) (mPEG-PCL) block copolymer and formulate Erlotinib HCl–loaded mPEG-PCL nanoparticles for enhancing the bioavailability of the drug. Using the ring-opening polymerization technique, PEGylated mPEG-PCL block copolymer was synthesized, and the structure of the copolymer was characterized using FTIR, 1H-NMR, and DSC techniques. The solvent evaporation approach was used to effectively encapsulate Erlotinib HCl within block copolymeric nanoparticles. Erlotinib HCl–loaded mPEG-PCL nanoparticles had a mean particle size of 146.5 ± 2.37 nm and a zeta potential of −27.8 ± 2.77 mV. The nanoparticles had a percent entrapment efficiency of 80.78 ± 0.09%. The in vitro drug release of Erlotinib HCl–loaded copolymeric nanoparticles showed a slow and sustained release behavior which could be maintained for up to 72 h. The Korsmeyer-Peppas fitting findings indicated that the drug release process followed a non-Fickian diffusion mechanism. The pharmacokinetic (PK) behavior of the developed nanoformulation was studied in albino Wistar rats, and the relative bioavailability of the optimized NP formulation given by intravenous route was found to be 187.33%. The PK data suggested that Erlotinib HCl–loaded mPEG-PCL copolymeric nanoparticles can dramatically alter the PK behavior of Erlotinib HCl and greatly improve the drug’s bioavailability by as much as three times when compared to the oral formulation. As a result, it was established that the block copolymeric nanoparticles have promise for the effective encapsulation of Erlotinib HCL for an injectable formulation with increased bioavailability.
Graphical Abstract









Similar content being viewed by others
Data Availability
Data are available upon request.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2016;66(1):7–30.
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–94.
Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer. 2014;14(8):535–46.
Crino L, Weder W, Van Meerbeeck J, Felip ES. Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21:103–15.
Van Erp NP, Gelderblom H, Guchelaar HJ. Clinical pharmacokinetics of tyrosine kinase inhibitors. Cancer Treat Rev. 2009;35(8):692–706.
Truong DH, Tran TH, Ramasamy T, Choi JY, Lee HH, Moon C, Choi HG, Yong CS, Kim JO. Development of solid self-emulsifying formulation for improving the oral bioavailability of erlotinib. AAPS PharmSciTech. 2016;17:466–73.
Cataldo VD, Gibbons DL, Pérez-Soler R, Quintás-Cardama A. Treatment of non–small-cell lung cancer with erlotinib or gefitinib. N Engl J Med. 2011;364(10):947–55.
Korgaonkar N, Yadav KS. Understanding the biology and advent of physics of cancer with perspicacity in current treatment therapy. Life Sci. 2019;239:117060.
Cohen MH, Johnson JR, Chattopadhyay S, Tang S, Justice R, Sridhara R, Pazdur R. Approval summary: erlotinib maintenance therapy of advanced/metastatic non-small cell lung cancer (NSCLC). J Oncol. 2010;15(12):1344–51.
Budha NR, Frymoyer A, Smelick GS, Jin JY, Yago MR, Dresser MJ, Holden SN, Benet LZ, Ware JA. Drug absorption interactions between oral targeted anticancer agents and PPIs: is pH-dependent solubility the Achilles heel of targeted therapy? Clin Pharm Therap. 2012;92(2):203–13.
Wakeling AE, Guy SP, Woodburn JR, Ashton SE, Curry BJ, Barker AJ, Gibson KH. ZD1839 (Iressa) an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res. 2002;62(20):5749–54.
Benet LZ. The role of BCS (biopharmaceutics classification system) and BDDCS (biopharmaceutics drug disposition classification system) in drug development. J Pharm Sci. 2013;102(1):34–42.
Bruinsmann FA, Buss JH, Souto GD, Schultze E, de Cristo Soares Alves A, Seixas FK, Collares TV, Pohlmann AR, Guterres SS. 2020. Erlotinib-loaded poly (ε-Caprolactone) nanocapsules improve in vitro cytotoxicity and anticlonogenic effects on human A549 lung cancer cells. AAPS PharmSciTech. 21:1-2.
Ling J, Fettner S, Lum BL, Riek M, Rakhit A. Effect of food on the pharmacokinetics of erlotinib, an orally active epidermal growth factor receptor tyrosine-kinase inhibitor, in healthy individuals. Anticancer drugs. 2008;19(2):209–16.
Reuter SE, Evans AM, Shakib S, Lungershausen Y, Francis B, Valentini G, Bacchieri A, Ubben D, Pace S. Effect of food on the pharmacokinetics of piperaquine and dihydroartemisinin. Clin Drug Investig. 2015;35:559–67.
D’Arcangelo M, Cappuzzo F. Erlotinib in the first-line treatment of non-small-cell lung cancer. Expert Rev Anticancer Ther. 2013;13(5):523–33.
Taiwade C, Fulfager A, Bhargave H, Soni G, Yadav K. Erlotinib hydrochloride novel drug delivery systems: a mini review unravelling the role of micro- and nanocarriers. Drug Deliv Lett. 2021;11(4):295–306.
Devasari N, Dora CP, Singh C, Paidi SR, Kumar V, Sobhia ME, Suresh S. Inclusion complex of erlotinib with sulfobutyl ether-β-cyclodextrin: preparation, characterization, in silico, in vitro and in vivo evaluation. Carbohydr Polym. 2015;134:547–56.
Vrignaud S, Hureaux J, Wack S, Benoit JP, Saulnier P. Design, optimization and in vitro evaluation of reverse micelle-loaded lipid nanocarriers containing erlotinib hydrochloride. Int J Pharm. 2013;436(1–2):194–200.
Marslin G, Sheeba CJ, Kalaichelvan VK, Manavalan R, Neelakanta Reddy P, Franklin G. Poly (D, L-lactic-co-glycolic acid) nanoencapsulation reduces Erlotinib-induced subacute toxicity in rat. J Biomed Nanotechnol. 2009;5(5):464–71.
Jesson G, Brisander M, Andersson P, Demirbüker M, Derand H, Lennernäs H, Malmsten M. Carbon dioxide-mediated generation of hybrid nanoparticles for improved bioavailability of protein kinase inhibitors. Pharm Res. 2014;31:694–705.
Morton SW, Lee MJ, Deng ZJ, Dreaden EC, Siouve E, Shopsowitz KE, Shah NJ, Yaffe MB, Hammond PT. A nanoparticle-based combination chemotherapy delivery system for enhanced tumor killing by dynamic rewiring of signaling pathways. Sci Signal. 2014;7(325):44.
Trummer BJ, Iyer V, Balu-Iyer SV, O’Connor R, Straubinger RM. Physicochemical properties of epidermal growth factor receptor inhibitors and development of a nanoliposomal formulation of gefitinib. J Pharm Sci. 2012;101(8):2763–76.
Truong DH, Le VK, Pham TT, Dao AH, Pham TP, Tran TH. Delivery of erlotinib for enhanced cancer treatment: an update review on particulate systems. Drug Deliv Sci Technol. 2020;55:101348.
Mandal B, Mittal NK, Balabathula P, Thoma LA, Wood GC. Development and in vitro evaluation of core–shell type lipid–polymer hybrid nanoparticles for the delivery of erlotinib in non-small cell lung cancer. Eur J Pharm Sci. 2016;81:162–71.
Dora CP, Trotta F, Kushwah V, Devasari N, Singh C, Suresh S, Jain S. Potential of erlotinib cyclodextrin nanosponge complex to enhance solubility, dissolution rate, in vitro cytotoxicity and oral bioavailability. Carbohydr Polym. 2016;137:339–49.
Patel K, Doddapaneni R, Patki M, Sekar V, Bagde A, Singh M. Erlotinib-valproic acid liquisolid formulation: evaluating oral bioavailability and cytotoxicity in erlotinib-resistant non-small cell lung cancer cells. AAPS PharmSciTech. 2019;20:1–1.
Phelps MA, Stinchcombe TE, Blachly JS, Zhao W, Schaaf LJ, Starrett SL, Wei L, Poi M, Wang D, Papp A, Aimiuwu J. Erlotinib in African Americans with advanced non–small cell lung cancer: a prospective randomized study with genetic and pharmacokinetic analyses. Clin Pharm Therap. 2014;96(2):182–91.
Li J, Zhao M, He P, Hidalgo M, Baker SD. Differential metabolism of gefitinib and erlotinib by human cytochrome P450 enzymes. Clin Cancer Res. 2007;13(12):3731–7.
Peters S, Zimmermann S, Adjei AA. Oral epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of non-small cell lung cancer: comparative pharmacokinetics and drug–drug interactions. Cancer Treat Rev. 2014;40(8):917–26.
Babu A, Templeton AK, Munshi A, Ramesh R. Nanodrug delivery systems: a promising technology for detection, diagnosis, and treatment of cancer. AAPS PharmSciTech. 2014;15:709–21.
Raut H, Jadhav C, Shetty K, Laxane N, Nijhawan HP, Rao GK, Alavala RR, Joshi G, Patro CN, Soni G, Yadav KS. Sorafenib tosylate novel drug delivery systems: implications of nanotechnology in both approved and unapproved indications. OpenNano. 2022;8:100103.
In GK, Nieva J. Emerging chemotherapy agents in lung cancer: nanoparticles therapeutics for non-small cell lung cancer. Transl Lung Cancer Res. 2015;4(4):340–55.
Cucinotto I, Fiorillo L, Gualtieri S, Arbitrio M, Ciliberto D, Staropoli N, Grimaldi A, Luce A, Tassone P, Caraglia M, Tagliaferri P. 2013. Nanoparticle albumin bound Paclitaxel in the treatment of human cancer: nanodelivery reaches prime-time?. J Drug Deliv. 2013.
Kim DW, Kim SY, Kim HK, Kim SW, Shin SW, Kim JS, Park K, Lee MY, Heo DS. Multicenter phase II trial of Genexol-PM, a novel Cremophor-free, polymeric micelle formulation of paclitaxel, with cisplatin in patients with advanced non-small-cell lung cancer. Ann Oncol. 2007;18(12):2009–14.
Autio KA, Dreicer R, Anderson J, Garcia JA, Alva A, Hart LL, Milowsky MI, Posadas EM, Ryan CJ, Graf RP, Dittamore R. Safety and efficacy of BIND-014, a docetaxel nanoparticle targeting prostate-specific membrane antigen for patients with metastatic castration-resistant prostate cancer: a phase 2 clinical trial. JAMA Oncol. 2018;4(10):1344–51.
Yadav KS, Sawant KK. Modified nanoprecipitation method for preparation of cytarabine-loaded PLGA nanoparticles. AAPS PharmSciTech. 2010;11:1456–65.
Danafar H. MPEG–PCL copolymeric nanoparticles in drug delivery systems. Cogent Med. 2016;3(1):1142411.
Yadav KS, Jacob S, Sachdeva G, Sawant KK. Intracellular delivery of etoposide loaded biodegradable nanoparticles: cytotoxicity and cellular uptake studies. J Nanosci Nanotechnol. 2011;11(8):6657–67.
Peng W, Jiang XY, Zhu Y, Omari-Siaw E, Deng WW, Yu JN, Xu XM, Zhang WM. Oral delivery of capsaicin using MPEG-PCL nanoparticles. Acta Pharm Sin B. 2015;36(1):139–48.
O’Reilly Beringhs A, Ndaya D, Bosire R, Kasi RM, Lu X. Stabilization and x-ray attenuation of PEGylated cholesterol/polycaprolactone-based perfluorooctyl bromide nanocapsules for CT imaging. AAPS PharmSciTech. 2021;22:1–3.
Manjili HK, Ghasemi P, Malvandi H, Mousavi MS, Attari E, Danafar H. Pharmacokinetics and in vivo delivery of curcumin by copolymeric mPEG-PCL micelles. Eur J Pharm Biopharm. 2017;116:17–30.
Nosrati H, Adinehvand R, Manjili HK, Rostamizadeh K, Danafar H. Synthesis, characterization, and kinetic release study of methotrexate loaded mPEG–PCL polymersomes for inhibition of MCF-7 breast cancer cell line. Pharm Dev Technol. 2019;24(1):89–98.
Manjili HR, Malvandi H, Mosavi MS, Danafar H. Preparation and physicochemical characterization of biodegradable mPEG-PCL core-shell micelles for delivery of artemisinin. Pharm Sci. 2016;22(4):234–43.
Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161(2):505–22.
Guo Z, Sui J, Ma M, Hu J, Sun Y, Yang L, Fan Y, Zhang X. pH-Responsive charge switchable PEGylated ε-poly-l-lysine polymeric nanoparticles-assisted combination therapy for improving breast cancer treatment. J Control Release. 2020;326:350–64.
Soni G, Yadav KS, Gupta MK. QbD based approach for formulation development of spray dried microparticles of erlotinib hydrochloride for sustained release. J Drug Deliv Sci Technol. 2020;57:101684.
Sharma A, Fish BL, Moulder JE, Medhora M, Baker JE, Mader M, Cohen EP. Safety and blood sample volume and quality of a refined retro-orbital bleeding technique in rats using a lateral approach. Lab Anim. 2014;43(2):63–6.
Sahu PK, Sharma A, Rayees S, Kour G, Singh A, Khullar M, Magotra A, Paswan SK, Gupta M, Ahmad I, Roy S. Pharmacokinetic study of piperine in Wistar rats after oral and intravenous administration. Int J Drug Deliv. 2014;6(1):82.
Zhou X, Tao H, Shi KH. Development of a nanoliposomal formulation of erlotinib for lung cancer and in vitro/in vivo antitumoral evaluation. Drug Des Devel Ther. 2017:1-8.
Wei W, Li S, Xu H, Zhou F, Wen Y, Song Z, Feng S, Feng R. MPEG-PCL copolymeric micelles for encapsulation of azithromycin. AAPS PharmSciTech. 2018;19:2041–7.
De Abreu LC, Todaro V, Sathler PC, da Silva LC, do Carmo FA, Costa CM, Toma HK, Castro HC, Rodrigues CR, de Sousa VP, Cabral LM. 2016. Development and characterization of nisin nanoparticles as potential alternative for the recurrent vaginal candidiasis treatment. AAPS PharmSciTech. 2016;17:1421-7.
Yadav KS, Mishra DK, Deshpande A, Pethe AM. Levels of drug targeting. In: Basic fundamentals of drug delivery. Academic Press; 2019. p. 269-305
Hussain Z, Khan S, Imran M, Sohail M, Shah SW, de Matas M. PEGylation: A promising strategy to overcome challenges to cancer-targeted nanomedicines: a review of challenges to clinical transition and promising resolution. Drug Deliv Transl Res. 2019;9:721–34.
Reich G. In vitro stability of poly (D, L-lactide) and poly (D, L-lactide)/poloxamer nanoparticles in gastrointestinal fluids. Drug Dev Ind Pharm. 1997;23(12):1191–200.
Yadav KS, Kale K. High pressure homogenizer in pharmaceuticals: understanding its critical processing parameters and applications. J Pharm Innov. 2020;15:690–701.
Girotra P, Singh SK, Kumar G. Development of zolmitriptan loaded PLGA/poloxamer nanoparticles for migraine using quality by design approach. Int J Biol Macromol. 2016;85:92–101.
Xie W, Zhu W, Shen Z. Synthesis, isothermal crystallization and micellization of mPEG–PCL diblock copolymers catalyzed by yttrium complex. Polym J. 2007;48(23):6791–8.
Bilati U, Allémann E, Doelker E. Development of a nanoprecipitation method intended for the entrapment of hydrophilic drugs into nanoparticles. Eur J Pharm Sci. 2005;24(1):67–75.
Peppas NA. Analysis of Fickian and non-Fickian drug release from polymers. Pharm Acta Helv. 1985;60(4):110–1.
Wibowo FR, Saputra OA, Lestari WW, Koketsu M, Mukti RR, Martien R. pH-triggered drug release controlled by poly (styrene sulfonate) growth hollow mesoporous silica nanoparticles. ACS omega. 2020;5(8):4261–9.
Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15(1):25–35.
Ritger PL, Peppas NA. A simple equation for description of solute release II Fickian and anomalous release from swellable devices. J Control Release. 1987;5(1):37–42.
Danyuo Y, Ani CJ, Salifu AA, Obayemi JD, Dozie-Nwachukwu S, Obanawu VO, Akpan UM, Odusanya OS, Abade-Abugre M, McBagonluri F, Soboyejo WO. Anomalous release kinetics of prodigiosin from poly-N-isopropyl-acrylamid based hydrogels for the treatment of triple negative breast cancer. Sci Rep. 2019;9(1):1–4.
Yadav KS, Jacob S, Sachdeva G, Chuttani K, Mishra AK, Sawant KK. Long circulating PEGylated PLGA nanoparticles of cytarabine for targeting leukemia. J Microencapsul. 2011;28(8):729–42.
Yadav KS, Chuttani K, Mishra AK, Sawant KK. Long circulating nanoparticles of etoposide using PLGA-MPEG and PLGA-pluronic block copolymers: characterization, drug-release, blood-clearance, and biodistribution studies. Drug Dev Res. 2010;71(4):228–39.
Kharwade R, More S, Suresh E, Warokar A, Mahajan N, Mahajan U. Improvement in bioavailability and pharmacokinetic characteristics of efavirenz with booster dose of ritonavir in PEGylated PAMAM G4 dendrimers. AAPS PharmSciTech. 202;23(6):177.
Kale K, Fulfager A, Juvale K, Yadav KS. Long circulating polymeric nanoparticles of gemcitabine HCl using PLGA-PEG-PPG-PEG block copolymer. Int J Polym Mater. 2022:1-4.
Yadav KS, Chuttani K, Mishra AK, Sawant KK. Effect of size on the biodistribution and blood clearance of etoposide-loaded PLGA nanoparticles. PDA J Pharm Sci Technol. 2011;65(2):131–9.
Yi S, Zhang C, Hu J, Meng Y, Chen L, Yu H, Li S, Wang G, Zheng G, Qiu Z. Preparation, characterization, and in vitro pharmacodynamics and pharmacokinetics evaluation of PEGylated urolithin A liposomes. AAPS PharmSciTech. 2021;22:1–2.
Torchilin VP, Trubetskoy VS. Which polymers can make nanoparticulate drug carriers –long-circulating? Adv Drug Deliv Rev. 1995;16(2–3):141–55.
Yin N, Yu H, Zhang X, Lv X. 2020 Enhancement of pancreatic cancer therapy efficacy by type-1 matrix metalloproteinase-functionalized nanoparticles for the selective delivery of gemcitabine and erlotinib. Drug Des Devel Ther. 2020:4465-75.
Xu H, He C, Liu Y, Jiang J, Ma T. Novel therapeutic modalities and drug delivery–erlotinib liposomes modified with galactosylated lipid: in vitro and in vivo investigations. Artif Cells Nanomed Biotechnol. 2018;46(8):1902–7.
Shen Y, Li W. 2018 HA/HSA co-modified erlotinib–albumin nanoparticles for lung cancer treatment. Drug Des Devel Ther. 2018:2285-92.
Acknowledgements
The authors would like to thank MSN Laboratories Pvt. Ltd., Formulation division, MSN House, Hyderabad, India, for the gift sample of Erlotinib HCl.
Author information
Authors and Affiliations
Contributions
Khushwant Yadav: conceptualization, methodology, investigation, writing, reviewing, and editing final manuscript
Hardik Bhargave: methodology, investigation, writing original draft
Harsh Nijhawan: methodology, validation, writing, reviewing, and editing the manuscript
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhargave, H., Nijhawan, H. & Yadav, K.S. PEGylated Erlotinib HCl Injectable Nanoformulation for Improved Bioavailability. AAPS PharmSciTech 24, 101 (2023). https://doi.org/10.1208/s12249-023-02560-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02560-5