Abstract
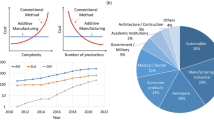
There has been a tremendous increase in the investigations of three-dimensional (3D) printing for biomedical and pharmaceutical applications, and drug delivery in particular, ever since the US FDA approved the first 3D printed medicine, SPRITAM® (levetiracetam) in 2015. Three-dimensional printing, also known as additive manufacturing, involves various manufacturing techniques like fused-deposition modeling, 3D inkjet, stereolithography, direct powder extrusion, and selective laser sintering, among other 3D printing techniques, which are based on the digitally controlled layer-by-layer deposition of materials to form various geometries of printlets. In contrast to conventional manufacturing methods, 3D printing technologies provide the unique and important opportunity for the fabrication of personalized dosage forms, which is an important aspect in addressing diverse patient medical needs. There is however the need to speed up the use of 3D printing in the biopharmaceutical industry and clinical settings, and this can be made possible through the integration of modern technologies like artificial intelligence, machine learning, and Internet of Things, into additive manufacturing. This will lead to less human involvement and expertise, independent, streamlined, and intelligent production of personalized medicines. Four-dimensional (4D) printing is another important additive manufacturing technique similar to 3D printing, but adds a 4th dimension defined as time, to the printing. This paper aims to give a detailed review of the applications and principles of operation of various 3D printing technologies in drug delivery, and the materials used in 3D printing, and highlight the challenges and opportunities of additive manufacturing, while introducing the concept of 4D printing and its pharmaceutical applications.







Similar content being viewed by others
Change history
10 March 2023
A Correction to this paper has been published: https://doi.org/10.1208/s12249-023-02542-7
References
Rahim TNAT, Abdullah AM, MdAkil H. Recent developments in fused deposition modeling-based 3D printing of polymers and their composites. Polym Rev. 2019;59(4):589–624.
Doestzada M, et al. Pharmacomicrobiomics: a novel route towards personalized medicine? Protein Cell. 2018;9(5):432–45.
Vaz VM, Kumar L. 3D Printing as a Promising Tool in Personalized Medicine. AAPS PharmSciTech. 2021;22(1):49.
Wang J, et al. Emerging 3D printing technologies for drug delivery devices: current status and future perspective. Adv Drug Deliv Rev. 2021;174:294–316.
Javaid M, Haleem A. 4D printing applications in medical field: a brief review. Clinical Epidemiology and Global Health. 2019;7(3):317–21.
Elbadawi M, McCoubrey LE, Gavins FKH, Ong JJ, Goyanes A, Gaisford S, Basit AW. Harnessing artificial intelligence for the next generation of 3D printed medicines. Adv Drug Deliv Rev. 2021;175: 113805.
Ong JJ, Castro BM, Gaisford S, Cabalar P, Basit AW, Perez G, Goyanes A. Accelerating 3D printing of pharmaceutical products using machine learning. Int J Pharm. 2022;4: 100120.
Elbadawi M, McCoubrey LE, Gavins FKH, Ong JJ, Goyanes A, Gaisford S, Basit AW. Disrupting 3D printing of medicines with machine learning. Trends Pharmacol Sci. 2021;42(9):745–57.
Castro BM, Elbadawi M, Ong JJ, Pollard T, Song Z, Gaisford S, Perez G, Basit AW, Cabalar P, Goyanes A. Machine learning predicts 3D printing performance of over 900 drug delivery systems. J Control Release. 2021;337:530–45.
Grof Z, Stepanek F. Artificial intelligence based design of 3D printed tablets for personalized medicine. Comput Chem Eng. 2021;154: 107492.
Banerjee A, Haridas HK, SenGupta A, Jabalia N. Artificial intelligence in 3D printing: a revolution in health care. Emerging Applications of 3D Printing During CoVID 19 Pandemic. 2022:57–79.
Tathe A, Ghodke M, Nikalje AP. A brief review: biomaterials and their apllication. Int J Pharm Pharm Sci. 2010;2(4):19–23.
Nikita N, Kamlesh W, Milind U. An overview on biomaterials: pharmaceutical and biomedical applications. Appl Sci. 2021;11(11):154–61.
Festas AJ, Ramos A, Davim JP. Medical devices biomaterials – a review. The Journal of Materials: Design and Applications. 2020;234(1):218–28.
Bandari S, Nyavanandi D, Dumpa N, Repka MA. Coupling hot melt extrusion and fused deposition modeling: critical properties for successful performance. Adv Drug Deliv Rev. 2021;172:52–63.
Cailleaux S, Sanchez-Ballester NM, Gueche YA, Bataille B, Soulairol I. Fused deposition modeling (FDM), the new asset for the production of tailored medicines. J Control Release. 2021;330:821–41.
Long J, Gholizadeh H, Lu J, Bunt C, Seyfoddin A. Applications of fused deposition modelling (FDM) method of 3D printing in drug delivery. Curr Pharm Des. 2017;23(3):433–9.
Sánchez-Guirales SA, Jurado N, Kara A, Lalatsa A, Serrano DR. Understanding direct powder extrusion for fabrication of 3d printed personalised medicines: a case study for nifedipine minitablets. Pharm. 2021;13(10).
Borandeh S, van Bochove B, Teotia A, Seppälä J. Polymeric drug delivery systems by additive manufacturing. Adv Drug Deliv Rev. 2021;173:349–73.
Goole J, Amighi K. 3D printing in pharmaceutics: a new tool for designing customized drug delivery systems. Int J Pharm. 2016;499(1–2):376–94.
Zheng Y, Deng F, Wang B, Wu Y, Luo Q, Zuo X, Li X. Melt extrusion deposition (MED™) 3D printing technology: a paradigm shift in design and development of modified release drug products. Int J Pharm. 2021;602.
Goyanes A, Allahham N, Trenfield SJ, Stoyanov E, Gaisford S, Basit AW. Direct powder extrusion 3D printing: Fabrication of drug products using a novel single-step process. Int J Pharm. 2019;567:118471.
AziziMachekposhti S, Mohaved S, Narayan RJ. Inkjet dispensing technologies: recent advances for novel drug discovery. Expert Opin Drug Discov. 2019;14(2):101–13.
Boehm RD, Miller PR, Daniels J, Stafslien S, Narayan RJ. Inkjet printing for pharmaceutical applications. Mater Today. 2014;17(5):247–52.
Daly R, Harrington TS, Martin GD, Hutchings IM. Inkjet printing for pharmaceutics - a review of research and manufacturing. Int J Pharm. 2015;494(2):554–67.
Derby B. Inkjet printing of functional and structural materials: fluid property requirements, feature stability, and resolution. Annu Rev Mater Res. 2010;40:395–414.
Hoath SD. Fundamentals of inkjet printing: the science of inkjet and droplets: John Wiley & Sons; 2016.
De Gans BJ, Duineveld PC, Schubert US. Inkjet printing of polymers: state of the art and future developments. Adv Mater. 2004;16(3):203–13.
Cader HK, et al. Water-based 3D inkjet printing of an oral pharmaceutical dosage form. Int J Pharm. 2019;564(April):359–68.
Genina N, et al. Tailoring controlled-release oral dosage forms by combining inkjet and flexographic printing techniques. Eur J Pharm Sci. 2012;47(3):615–23.
Edinger M, Bar-Shalom D, Sandler N, Rantanen J, Genina N. QR encoded smart oral dosage forms by inkjet printing. Int J Pharm. 2018;536(1):138–45.
Melendez PA, Kane KM, Ashvar CS, Albrecht M, Smith PA. Thermal inkjet application in the preparation of oral dosage forms: dispensing of prednisolone solutions and polymorphic characterization by solid-state spectroscopic techniques. J Pharm Sci. 2012;101(7):2271–80.
Buanz ABM, Saunders MH, Basit AW, Gaisford S. Preparation of personalized-dose salbutamol sulphate oral films with thermal ink-jet printing. Pharm Res. 2011;28(10):2386–92.
Planchette C, et al. Printing medicines as orodispersible dosage forms: effect of substrate on the printed micro-structure. Int J Pharm. 2016;509(1–2):518–27.
Vuddanda PR, et al. Personalisation of warfarin therapy using thermal ink-jet printing. Eur J Pharm Sci. 2018;117(September 2017):80–7.
Kollamaram G, Hopkins SC, Glowacki BA, Croker DM, Walker GM. Inkjet printing of paracetamol and indomethacin using electromagnetic technology: rheological compatibility and polymorphic selectivity. Eur J Pharm Sci. 2018;115(September2017):248–57.
Sandler N, et al. Inkjet printing of drug substances and use of porous substrates-towards individualized dosing. J Pharm Sci. 2011;100(8):3386–95.
Genina N, Janßen EM, Breitenbach A, Breitkreutz J, Sandler N. Evaluation of different substrates for inkjet printing of rasagiline mesylate. Eur J Pharm Biopharm. 2013;85(3 PART B):1075–83.
Park T-M, Kang D, Jang I, Yun W-S, Shim J-H, Jeong YH, et al. Fabrication of in vitro cancer microtissue array on fibroblast-layered nanofibrous membrane by inkjet printing. Int J Mol Sci. 2017;18(11):2348.
Faulkner-Jones A, Fyfe C, Cornelissen D-J, Gardner J, King J, Courtney A, et al. Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D. Biofabrication. 2015;7(4):044102.
Xu T, Zhao W, Zhu JM, Albanna MZ, Yoo JJ, Atala A. Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials. 2013;34(1):130–9.
Xu T, Binder KW, Albanna MZ, Dice D, Zhao W, Yoo JJ, et al. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication. 2012;5(1):015001.
Skardal A, et al. Bioprinted amniotic fluid-derived stem ccells accelerate healing of large skin wounds. Stem Cells Transl Med. 2012;1(11):792–802.
Acosta-Vélez GF, Linsley CS, Craig MC, Wu BM. Photocurable bioink for the inkjet 3D pharming of hydrophilic drugs. Bioeng. 2017;4(1).
Wickström H, et al. Improvement of dissolution rate of indomethacin by inkjet printing. Eur J Pharm Sci. 2015;75:91–100.
Scoutaris N, Alexander MR, Gellert PR, Roberts CJ. Inkjet printing as a novel medicine formulation technique. J Control Release. 2011;156(2):179–85.
Inzana JA, Olvera D, Fuller SM, Kelly JP, Graeve OA, Schwarz EM, Kates SL, Awad HA. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials. 2014;35:4026–34.
Goyanes A, Det-Amornrat U, Wang J, Basit AW, Gaisford S. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J Control Release. 2016;234:41–8.
Anadioti E, Kane B, Soulas E. Current and emerging applications of 3D printing in restorative dentistry. Curr Oral Heal Reports. 2018;5(2):133–9.
Tamay DG, Dursun Usal T, Alagoz AS, Yucel D, Hasirci N, Hasirci V. 3D and 4D printing of polymers for tissue engineering applications. Front Bioeng Biotechnol. 2019;7:164.
Bird D, Eker E, Ravindra NM, editors. 3D printing of pharmaceuticals and transdermal drug delivery––an overview. TMS 2019 148th Annual Meeting & Exhibition Supplemental Proceedings; 2019: Springer.
Rodríguez-Pombo L, Xu X, Seijo-Rabina A, Ong JJ, Alvarez-Lorenzo C, Rial C, Goyanes A. Volumetric 3D printing for rapid production of medicines. Addit Manuf. 2022;52: 102673.
Lim SH, Kathuria H, Tan JJY, Kang L. 3D printed drug delivery and testing systems: a passing fad or the future? Adv Drug Deliv Rev. 2018;132:139–68.
Economidou SN, Lamprou DA, Douroumis D. 3D printing applications for transdermal drug delivery. Int J Pharm. 2018;544(2):415–24.
Karakurt I, Lin L. 3D printing technologies: techniques, materials, and post-processing. Curr Opin Chem Eng. 2020;28:134–43.
Heshmati NA, Zhang Y, Wang J, Lu A, Pillai AR, Maniruzzaman M. A novel 3D printing particulate manufacturing technology for encapsulation of protein therapeutics: Sprayed multi adsorbed-droplet reposing technology (SMART). Bioengineering. 2022;9(11):653.
Agrawal A, Gupta AK. 3D Printing technology in pharmaceuticals and biomedical: a review. Journal of Drug Delivery and Therapeutics. 2019;9(2):1–4.
Sen K, Mehta T, Sansare S, Sharifi L, Ma AW, Chaudhuri B. Pharmaceutical applications of powder-based binder jet 3D printing process: a review. Adv Drug Deliv Rev. 2021;177: 113943.
Fina F, Goyanes A, Gaisford S, Basit AW. Selective laser sintering (SLS) 3D printing of medicines. Int J Pharm. 2017;529(1–2):285–93.
Singh S, Ramakrishna S, Berto F. 3D printing of polymer composites: a short review. Mater Des Process Commun. 2020;2(2):1–13.
Charoo NA, et al. Selective laser sintering 3D printing–an overview of the technology and pharmaceutical applications. Drug Dev Ind Pharm. 2020;46(6):869–77.
Manoharan V, Chou SM, Forrester S, Chai GB, Kong PW. Application of additive manufacturing techniques in sports footwear: this paper suggests a five-point scoring technique to evaluate the performance of four AM techniques, namely, stereolithography (SLA), PolyJet (PJ), selective laser sintering (SLS) and t. Virtual Phys Prototyp. 2013;8(4):249–52.
Awad A, Fina F, Goyanes A, Gaisford S, Basit AW. Advances in powder bed fusion 3D printing in drug delivery and healthcare. Adv Drug Deliv Rev. 2021;174:406–24.
Cui M, et al. Exploration and preparation of patient-specific ciprofloxacin implants drug delivery system via 3D printing technologies. J Pharm Sci. 2021;110(11):3678–89.
Farmer ZL, et al. 3D printed estradiol-eluting urogynecological mesh implants: Influence of material and mesh geometry on their mechanical properties. Int J Pharm. 2020;593(October):2021.
Yang Y, et al. 3D-printed polycaprolactone-chitosan based drug delivery implants for personalized administration. Mater Des. 2022;214: 110394.
Wu L, Park J, Kamaki Y, Kim B. Optimization of the fused deposition modeling-based fabrication process for polylactic acid microneedles. Microsyst Nanoeng. 2021;7(1).
Krause J, Müller L, Sarwinska D, Seidlitz A, Sznitowska M, Weitschies W. 3D printing of mini tablets for pediatric use. Pharmaceuticals. 2021;14(2):1–16.
Ayyoubi S et al. 3D printed spherical mini-tablets: geometry versus composition effects in controlling dissolution from personalised solid dosage forms. Int J Pharm. 2021;597(January).
Nukala PK, Palekar S, Patki M, Patel K. Abuse deterrent immediate release egg-shaped tablet (Egglets) using 3D printing technology: quality by design to optimize drug release and extraction. AAPS PharmSciTech. 2019;20:1–12.
Okwuosa TC, Soares C, Gollwitzer V, Habashy R, Timmins P, Alhnan MA. On demand manufacturing of patient-specific liquid capsules via co-ordinated 3D printing and liquid dispensing. Eur J Pharm Sci. 2018;118(December 2017):134–43.
Bi M, Kyad A, Alvarez-Nunez F, Alvarez F. Enhancing and sustaining AMG 009 dissolution from a bilayer oral solid dosage form via microenvironmental pH modulation and supersaturation. AAPS PharmSciTech. 2011;12(4):1401–6.
Keikhosravi N, Mirdamadian SZ, Varshosaz J, Taheri A. Preparation and characterization of polypills containing aspirin and simvastatin using 3D printing technology for the prevention of cardiovascular diseases. Drug Dev Ind Pharm. 2020;46(10):1665–75.
Zhao X, Wei W, Niu R, Li Q, Hu C, Jiang S. 3D printed intragastric floating and sustained-release tablets with air chambers. J Pharm Sci. 2022;111(1):116–23.
Lamichhane S, Park J, Sohn DH, Lee S. Customized novel design of 3D printed pregabalin tablets for intra-gastric floating and controlled release using fused deposition modeling. 2019.
Fanous M, et al. Development of immediate release 3D-printed dosage forms for a poorly water-soluble drug by fused deposition modeling: Study of morphology, solid state and dissolution. Int J Pharm. 2021;599:120417.
Crișan AG, et al. QbD guided development of immediate release FDM-3D printed tablets with customizable API doses. Int J Pharm. 2021;613(October):2022.
Than YM, Titapiwatanakun V. Tailoring immediate release FDM 3D printed tablets using a quality by design (QbD) approach. Int J Pharm. 2021;599(January):120402.
Omari S, Ashour EA, Elkanayati R, Alyahya M, Almutairi M, Repka MA, MA. Formulation development of loratadine immediate-release tablets using hot-melt extrusion and 3D printing technology. J Drug Deliv Sci Technol. 2022;74:103505.
Elkanayati R, Chambliss WG, Omari S, Almutairi M, Repka MA, Ashour EA. Mucoadhesive buccal films for treatment of xerostomia prepared by hot-melt extrusion and 3D printing technologies. Journal of Drug Delivery Science and Technology. 2022;75: 103660.
Nashed N, Lam M, Nokhodchi A. A comprehensive overview of extended release oral dosage forms manufactured through hot melt extrusion and its combination with 3D printing. Int J Pharm. 2021;596(January):120237.
Đuranović M, Obeid S, Madžarević M, Cvijić S, Ibrić S. Paracetamol extended release FDM 3D printlets: evaluation of formulation variables on printability and drug release. Int J Pharm. 2020;592(October):2021.
Gültekin HE, Tort S, Tuğcu-Demiröz F, Acartürk F. 3D printed extended release tablets for once daily use: an in vitro and in vivo evaluation study for a personalized solid dosage form. Int J Pharm. 2020;596(October):2021.
Gorkem Buyukgoz G, Soffer D, Defendre J, Pizzano GM, Davé RN. Exploring tablet design options for tailoring drug release and dose via fused deposition modeling (FDM) 3D printing. Int J Pharm. 2020;591(October).
Vo AQ, Zhang J, Nyavanandi D, Bandari S, Repka MA. Hot melt extrusion paired fused deposition modeling 3D printing to develop hydroxypropyl cellulose based floating tablets of cinnarizine. Carbohydr Polym. 2020;246(May):116519.
Oladeji SA, Dadou SM, Zhao M, Li S, Jones DS, Andrews GP. The development and optimisation of gastro-retentive floating tablets using fused deposition modelling 3D printing. J Pharm Pharmacol. 2022;no. March, pp. 1–17.
Li R, Pan Y, Chen D, Xu X, Yan G, Fan T. Design, preparation and in vitro evaluation of core–shell fused deposition modelling 3D-printed verapamil hydrochloride pulsatile tablets. Pharmaceutics. 2022;14(2):437.
Tranová T, et al. Fused deposition modeling as a possible approach for the preparation of orodispersible tablets. Pharmaceuticals. 2022;15(1):69.
Manini G, Benali S, Mathew A, Napolitano S, Raquez JM, Goole J. Paliperidone palmitate as model of heat-sensitive drug for long-acting 3D printing application. Int J Pharm. 2022;618(December 2021):121662.
Mirdamadian SZ, Varshosaz J, Minaiyan M, Taheri A. 3D printed tablets containing oxaliplatin loaded alginate nanoparticles for colon cancer targeted delivery. An in vitro/in vivo study. Int J Biol Macromol. 2022;205(January):90–109.
Shi K, Slavage JP, M.Maniruzzaman M, Nokhodchi A. Role of release modifiers to modulate drug release from fused deposition modelling (FDM) 3D printed tablets. Int J Pharm. 2021;597(January).
Isreb A, Baj K, Wojsz M, Isreb M, Peak M, Alhnan MA. 3D printed oral theophylline doses with innovative ‘radiator-like’ design: impact of polyethylene oxide (PEO) molecular weight. Int J Pharm. 2019;564(November 2018):98–105.
Alhijjaj M, Belton P, Qi S. An investigation into the use of polymer blends to improve the printability of and regulate drug release from pharmaceutical solid dispersions prepared via fused deposition modeling (FDM) 3D printing. Eur J Pharm Biopharm. 2016;108:111–25.
Kempin W et al. Immediate release 3D-printed tablets produced via fused deposition modeling of a thermo-sensitive drug. Pharm Res. 2018;35(6).
Verstraete G, et al. 3D printing of high drug loaded dosage forms using thermoplastic polyurethanes. Int J Pharm. 2018;536(1):318–25.
Scoutaris N, Ross S, Douroumis D. Current trends on medical and pharmaceutical applications of inkjet printing technology. Pharm Res. 2016;33(8):1799–816.
Zakeri-Milani P, Nezhadi SH, Barzegar-Jalali M, Mohammadi L, Nokhodchi A, Valizadeh H. Studies on dissolution enhancement of prednisolone, a poorly water-soluble drug by solid dispersion technique. Adv Pharm Bull. 2011;1(1):48–53.
Iftimi LD, Edinger M, Bar-Shalom D, Rantanen J, Genina N. Edible solid foams as porous substrates for inkjet-printable pharmaceuticals. Eur J Pharm Biopharm. 2019;136(June 2018):38–47.
Eleftheriadis G, et al. Development and characterization of inkjet printed edible films for buccal delivery of b-complex vitamins. Pharmaceuticals. 2020;13(9):1–17.
He Y, et al. A reactive prodrug ink formulation strategy for inkjet 3D printing of controlled release dosage forms and implants. Adv Ther. 2020;3(6):1900187.
Palo M, et al. Development of oromucosal dosage forms by combining electrospinning and inkjet printing. Mol Pharm. 2017;14(3):808–20.
Kyobula M, et al. 3D inkjet printing of tablets exploiting bespoke complex geometries for controlled and tuneable drug release. J Control Release. 2017;261(March):207–15.
Acosta-Vélez GF, Zhu TZ, Linsley CS, Wu BM. Photocurable poly(ethylene glycol) as a bioink for the inkjet 3D pharming of hydrophobic drugs. Int J Pharm. 2018;546(1–2):145–53.
Fina F, et al. 3D printing of drug-loaded gyroid lattices using selective laser sintering. Int J Pharm. 2018;547(1–2):44–52.
Barakh Ali SF, et al. Understanding the effects of formulation and process variables on the printlets quality manufactured by selective laser sintering 3D printing. Int J Pharm. 2019;570(May):118651.
Allahham N, et al. Selective laser sintering 3D printing of orally disintegrating printlets containing ondansetron. Pharmaceutics. 2020;12(2):1–13.
Thakkar R, Jara MO, Swinnea S, Pillai AR, Maniruzzaman M. Impact of laser speed and drug particle size on selective laser sintering 3D printing of amorphous solid dispersions. Pharmaceutics. 2021;13(8):1149.
Mohamed EM, Barakh ASF, Rahman Z, Dharani S, Ozkan T, Kuttolamadom MA, Khan MA. Formulation optimization of selective laser sintering 3D-printed tablets of clindamycin palmitate hydrochloride by response surface methodology. AAPS PharmSciTech. 2020;21(6):1–15.
Xu X, et al. Stereolithography (SLA) 3D printing of an antihypertensive polyprintlet: case study of an unexpected photopolymer-drug reaction. Addit Manuf. 2020;33(December 2019):101071.
Robles-Martinez P et al. 3D printing of a multi-layered polypill containing six drugs using a novel stereolithographic method. Pharm. 2019;11(6).
Asikainen S, van Bochove B, Seppälä JV. Drug-releasing biopolymeric structures manufactured via stereolithography. Biomed Phys Eng Express. 2019;5(2):025008.
Pistone M, Racaniello GF, Arduino I, Laquintana V, Lopalco A, Cutrignelli A, et al. Direct cyclodextrin-based powder extrusion 3D printing for one-step production of the BCS class II model drug niclosamide. Drug Deliv Transl Res. 2022;12(8):1895–910.
Mendibil X, Tena G, Duque A, Uranga N, Campanero MÁ, Alonso J. Direct powder extrusion of paracetamol loaded mixtures for 3d printed pharmaceutics for personalized medicine via low temperature thermal processing. Pharmaceutics. 2021;13(6):907.
El Aita I, Rahman J, Breitkreutz J, Quodbach J. 3D-printing with precise layer-wise dose adjustments for paediatric use via pressure-assisted microsyringe printing. Eur J Pharm Biopharm. 2020;157(October):59–65.
Hu Y, et al. Botanical-inspired 4D printing of hydrogel at the microscale. Adv Funct Mater. 2020;30(4):1–10.
Ibrahim O (July 28, 2022), "Five companies personalizing treatments with 3D printed drugs," LABIOTECH.eu, https://www.labiotech.eu/best-biotech/five-companies-personalizing-treatments-with-3d-printed-drugs/
Balfour H (September 14, 2021). 3D printing - current pharmaceutical applications and future directions, EPR, https://www.europeanpharmaceuticalreview.com/article/162544/3d-printing-current-pharmaceutical-applications-and-future-directions/
Author information
Authors and Affiliations
Contributions
Derick Muhindo: design the review article, interpret the relevant literature, and write and review the original draft. Rasha Elkanayati: design the review article, interpret the relevant literature, and write and review the original draft. Priyanka Srinivasan: design the review article, interpret the relevant literature, and write and review the original draft. Michael A. Repka: critically revise the review article. Eman A. Ashour: select the topic of the review article, supervise, and critically revise the review article.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Muhindo, D., Elkanayati, R., Srinivasan, P. et al. Recent Advances in the Applications of Additive Manufacturing (3D Printing) in Drug Delivery: A Comprehensive Review. AAPS PharmSciTech 24, 57 (2023). https://doi.org/10.1208/s12249-023-02524-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02524-9