Abstract
The aim of this study was the improvement of rutin solubility along with targeting its release to colon for effective treatment of colon cancer. Five formulations of compression-coated tablets were prepared with the same core composition including rutin-polyvinyl pyrrolidone K30 solid dispersion (rutin-PVP K30 SD) but differ in being coated with either frankincense alone or different combinations of frankincense with gelatin. The superior formula was selected based on the in vitro drug release then further evaluated in terms of physical properties and in vivo performance in dogs using X-ray. Moreover, in vitro cytotoxicity of rutin, rutin-PVP K30 SD, frankincense, and a mixture of rutin-PVP K30 SD with frankincense in a ratio representing their concentrations in the selected formula was assessed against human colon cancer (HCT-116) cell lines using sulforhodamine B assay. The formula (F4) with the coat consisted of 65%w/w frankincense and 35%w/w gelatin achieved acceptable in vitro controlled drug release. In vivo X-ray in dogs confirmed that F4 tablet could remain intact in the stomach and small intestine until reaching the colon. In vitro cytotoxicity revealed that mixture of rutin-PVP K30 SD with frankincense was more effective in arresting cancer cell growth than rutin or frankincense alone. Moreover, stability studies revealed that F4 tablets were physically and chemically stable. Thus, improving rutin solubility using solid dispersion technique and formulating it into frankincense-based compression-coated (F4) tablets would be a successful approach for colonic delivery of rutin with potential of improving therapeutic efficacy.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colon cancer is ranked the third among the most common cancers globally and the second type of cancer causing mortality worldwide[1]. This disease is predicted to strike 3.2 million people in 2040 all over the world [2]. The major limitations of conventional chemotherapies used in treatment of colon cancer are low efficacy, toxicity, and high cost [3]. Rutin is a dietary flavonoid found in buckwheat seed, fruits, and fruit rinds, especially in citrus fruits like orange, grapefruit, and lemon [4]. Several in vitro studies on human cancer cell lines revealed that rutin could be a potential candidate for treatment of colon cancer through arresting cell cycle, and triggering apoptosis, as well as inhibiting proliferation, angiogenesis, and/or metastasis [5,6,7,8,9,10]. Moreover, rutin has anti-inflammatory and anti-oxidant effects [11]. However, the poor aqueous solubility of rutin (0.125 mg/ml) affects the drug in vivo performance [12]. Various systems have been developed and studied for rutin delivery including nanocrystals [13], nanostructured liquisolid systems [14], phytosomes [15], prenanoemulsion [16], solid lipid nanoparticles [17], metallic nanoparticles [18], polymeric nanoparticles [19], pH-sensitive nanospheres [20], and cyclodextrin inclusion complexes [21,22,23]. However, the incorporation of rutin into a solid dispersion with a hydrophilic polymer could be a simpler approach compared to the previously mentioned ones and was reported to be greatly successful in increasing rutin solubility and dissolution rate [24]. Moreover, the adaptation of colon targeted drug delivery approach is useful in directing drugs to cancer cells in colon, which enhances therapeutic efficacy. Currently, polymers of natural origin have been adopted in pharmaceutical research and used for modulated drug release delivery systems [25]. The resinous matter from the trees of the genus Boswellia (frankincense) is a natural lipophilic polymer [26]. Boswellia carterii is one of four species of the genus Boswellia and it is found in east Africa and China. Modern studies reported various pharmacological activities (e.g., anti-inflammatory, anti-microbial, and anti-tumor) for the extracts and isolated compounds of Boswellia carterii [27]. The chemical composition of frankincense is 60–85% resins, 6–30% gums, and 5–9% essential oil [28]. Resin portion is composed of various triterpenes among which boswellic acids are the key ingredients for the pharmacological activity. The most important boswellic acid is acetyl-11-keto-β-boswellic acid [26, 29]. Some reported studies revealed the ability of Frankincense to retard drug release in controlled drug delivery systems [26, 30, 31]. In addition, Frankincense was found to possess antitumor activity against human colon cancer [32,33,34]. Hence, this study aimed to maximize the therapeutic efficacy of rutin through both improving its solubility and targeting its release to colon. Thus, frankincense was used as a compression coat for rutin-PVP K30 solid dispersion core tablets and evaluated for its potential in colonic targeted delivery and enhancing cytotoxic efficacy of rutin.
Materials and Methods
Materials
Chemicals
Rutin Tri hydrate was purchased from Oxford Lab Fine Chem. Llp, India. Frankincense (Boswellia carterii) resin was purchased from Harraz Herbs Company Cairo, Egypt, and was kindly authenticated as the resin of Boswellia carterii Birdwood (Somalia), Family Burseraceae, by Prof. Dr. Mohammed El Gebally, Professor of botanic plants, National Research Center, Egypt, and Al-Orman Botanical Garden Herbarium, Egypt. Polyvinylpyrrolidone (PVP K30) and barium sulfate were obtained from Sigma-Aldrich company, Germany. Avicel®PH 101 powder was purchased from Fluka Biochemika Company, Swizerland. Gelatin powder type A was obtained from Alpha Chemika Company, India. Sodium starch glycolate (SSG) was kindly donated as a gift by Epico Company, Egypt. Magnesium stearate, monobasic sodium phosphate, and dibasic sodium phosphate were obtained from El-Nasr Pharmaceutical Chemicals Company, Egypt. Skimmed milk (Miro skimmed powder milk) was manufactured by Arab Cultivators El-Tanbouli & Co, Sadat City, Egypt. All other chemicals are of analytical grade.
Cell Culture
Human colon carcinoma cell lines (HCT-116) used in this study were obtained from the American Type Culture Collection (ATCC, USA). The tumor cell lines were maintained at the cancer cell line special unit, National Cancer Institute, Cairo, Egypt.
Methods
Preparation of Rutin Solid Dispersions Using Solvent Evaporation Method
Solid dispersions (SDs) of rutin with a hydrophilic carrier either skimmed milk or PVP K30 were prepared in a weight ratio 1:1 using solvent evaporation method. A weighed amount of rutin was dissolved in 10 ml methanol, then an equal amount of skimmed milk or PVP K30 was added and the mixture was stirred using a magnetic stirrer for 30 min. The mixture was exposed to air to allow methanol evaporation until complete dryness. The powder residue was scratched to obtain SD then stored in dissector for further use [35].
Determination of Saturation Solubility of Pure Rutin and the Prepared SDs of Rutin
The solubility of pure rutin, rutin:skimmed milk SD (1:1w/w), and rutin:PVP K30 SD (1:1w/w) in 0.1 N HCl (pH 1.2) (simulated gastric fluid) and phosphate buffer (pH 7.4) (simulated colonic fluid) was determined by adding a known excess amount of drug (20 mg pure drug form or SD equivalent to 20 mg drug) to 5 ml of 0.1 N HCl (pH 1.2) or phosphate buffer pH 7.4 in capped glass test tubes. The tubes were equilibrated at 37 ± 0.5°C for 24 h in a thermostatically controlled water bath shaker (AHAAM Shaker Incubator with heating and light, Model 25, Egypt). The suspensions were filtered using 0.45-µm syringe filter. One milliliter of the filtrate was diluted appropriately and measured against blank for the determination of rutin concentration spectrophotometrically in 0.1 N HCl (pH 1.2) and phosphate buffer (pH 7.4) at λmax 255and 268 nm, respectively.
Determination of Swelling Percent of Frankincense Powder
The swelling percent was determined in water, 0.1 N HCl, and phosphate buffer pH 7.4. An accurately weighed 1 g of frankincense powder was placed in 100 ml glass-stoppered measuring cylinders then 25 ml of either water, 0.1 N HCl, and phosphate buffer pH 7.4 was added in each measuring cylinder and the mixture was vigorously shaken every 10 min for 1 h and allowed to stand for 3 h at room temperature. The experiment was done in triplicates for each liquid. Then frankincense powder was collected from each cylinder and weighed. Excess liquid was removed gently with a filter paper and the swelling ratio was estimated according to the following Eq. [36].
where w2 is the weight of the swollen material and w1 is the initial weight of the dry material.
Differential Scanning Calorimetry
The differential scanning calorimetry (DSC) analysis was carried out on pure drug, pure excipients, rutin-PVP K30 sd, and the physical mixture of equal weights of rutin-PVP K30 SD and all the other used ingredients. The analysis was performed using (Shimadzu DSC-50, Tokyo, Japan) instrument equipped with a computerized data station to detect any interaction. Samples (4 mg) were weighed accurately, inserted in standard aluminum pan, and heated at a constant heating rate of 10 °C/min from 25 to 300°C under nitrogen gas flow rate of 25 ml/min.
Fourier Transform Infrared Spectroscopy
The presence of any interaction of rutin with frankincense, PVP K30, Avicel® PH101, and gelatin was detected by infrared spectroscopy (JASCO 6100, Tokyo, Japan). The FTIR analysis was carried out on pure drug, pure excipients, rutin-PVP K30 SD, and the physical mixture of equal weights of rutin-PVP K30 SD and all the other used ingredients. Samples were grinded with about 100 mg dry KBr powder and compressed into transparent disc. The Fourier transform infrared spectroscopy (FT-IR) spectra were recorded for pure drug, pure excipients, drug–excipient physical mixtures (1:1w/w), solid dispersion of rutin with PVP K30, and the physical mixture of equal weights of rutin and all the used ingredients.
Preparation of Rutin Compression-Coated Tablets
Preparation of Core Tablets
The core tablets of rutin SD were prepared by direct compression of 100 mg rutin-PVP K30 SD (equivalent to a dose of 50 mg rutin) with Avicel® PH101 as a filler, SSG as a disintegrant, and magnesium stearate as a lubricant according to the amounts shown in Table I. The powders were homogenously mixed then the mixture was fed manually into 6-mm round die equipped with concave faced punch and compressed into tablets at constant compression force of 40 bar using a hydraulic press (PerkinElmer, Waltham, MA, USA).
Preparation of Coated Tablets
Half the amount of the coating materials was manually fed in 10-mm die cavity. Then, the core tablet was inserted in the center of the die cavity, followed by adding the remaining amount of the coat preparation. Finally the coated tablets were compressed by a concave-faced punch at constant applied force of 80 bar using a hydraulic press (PerkinElmer, Waltham, MA, USA). The total coat weight was 220 mg and the total weight for core and coat tablet was 350 mg for all the prepared formulae (F1–F5). The prepared compression-coated tablets were cylindrical in shape. The composition of core and coating materials used in preparation of five tablets formulae is shown in Table I.
In Vitro Release Studies of Rutin from the Prepared Tablets
The in vitro release of rutin from the prepared compression-coated tablets was performed using USP I apparatus (basket model) at a rotation speed of 100 rpm and a constant temperature of 37 ± 0.5°C. The release medium was 750 ml of 0.1 N HCl (pH 1.2) for 2 h followed by 500 ml phosphate buffer (pH 7.4) until the end of the experiment. Samples (4 mL) were withdrawn at 1-, 2-, 3-, 4-, 5-, 6-, 8-, 10-, 12-, and 24-h time intervals and replaced with an equal volume of fresh medium. The samples were filtered using 0.45-μm syringe filter and analyzed spectrophotometrically for rutin content at λ max255 nm for 0.1 N HCl (pH 1.2) release medium and at λ max268 nm for phosphate buffer (pH 7.4) release medium. The release studies were carried out in triplicate and the mean values were plotted as percentage cumulative drug released against time.
In vitro Release Kinetics and Mechanisms
The obtained in vitro release data were fitted to zero-order, first-order, and Higuchi equations to elucidate the kinetics of rutin release from the prepared compression-coated tablets [37, 38]. The proper release model was assessed on the basis of the regression coefficient (R2). Release model having highest R2 was selected as the best fit model.
To study the drug release mechanism, data obtained from in vitro release were analyzed also according to Korsmeyer–Peppas release model [39]. Given by the equation:
where (Mt) is the amount of drug released at time t, (M∞) is the amount of drug released at infinite time, (K) is the kinetic constant, and (n) is the diffusional exponent that indicates the release mechanism. The (n) values used for elucidation of drug release mechanism from the prepared compression-coated tablets were determined from the slope of the plot of log cumulative % of drug release (≤ 60%) versus log time. The (n) value depends on the release mechanism and the shape of the drug delivery device [40]. For cylindrical tablets, if (n ≤ 0.45), the release mechanism follows “Fickian diffusion” (case I). Values of (0.45 < n < 0.89) indicate non-Fickian model (anomalous transport) where release is controlled by a combination of diffusion and polymer relaxation. If the n value is 0.89, the drug release follows zero-order drug release (case II transport) where the drug release rate is independent on time and involves polymer relaxation. For the values of n higher than 0.89, the mechanism of drug release is regarded as super case II transport (relaxation) [41].
Characterization of the Selected (F4) Tablets
Determination of the Flow Properties of the Selected (F4) Formula
The powders of core and coating materials of the selected (F4) compression-coated tablets were evaluated for angle of repose, bulk density, tapped density, compressibility index, and Hausner ratio.
Angle of Repose
The angle of repose is the internal angle between the surface of the powder pile and the horizontal surface. Angle of repose is related to the coefficient of friction between the powder particles. The angle of repose was determined by the funnel method. The powder mixture of either core or coat of F4 was allowed to flow through the funnel freely onto the surface. The diameter of the powder cone was measured and angle of repose was calculated using the following Eq. [42].
where “h” and “r” are the height and radius of the cone, respectively. Three determinations were performed.
Powders that have angle of repose values of 25–30° are excellent in flow. Angle of repose values of 31–35° indicate good flow while values that exceed 40° indicate bad flow [43].
Bulk Density
The bulk density of a powder is the ratio of the mass of an untapped powder sample to its volume including the interparticle void volume.
For determining the bulk density of the core and coating powders, 3 g of either core or coat powders in same proportions as in F4 Formula were weighed. Then the powder of each mixture was poured into a graduated measuring cylinder then, the volume was measured.
Then the bulk density in (g/ml) was calculated using this equation:
where m is the mass of the powder and V0 is the apparent volume [43].
Tapped Density
The tapped density is obtained by manually tapping a graduated measuring cylinder containing the powder sample and volume readings are taken until insignificant further volume change is noticed.
where m is the mass of the powder and Vt is the tapped volume [43].
Compressibility Index (%)
Compressibility index (%) was determined directly from the following equation:
where ρ tapped is the tapped density and ρ bulk is the bulk density.
Powders show an excellent flowability when a compressibility index (%) is less than or equal 10% and a good flowability when a compressibility index (%) is from 11 to 15% [43].
Hausner’s Ratio
Hausner’s ratio can be calculated by using following equation:
Powders show an excellent flowability when Hausner’s ratio is from 1.00 to 1.11 and a good flowability when Hausner’s ratio is from 1.12 to 1.18[43].
Uniformity of Thickness
The thickness of tablets was determined using a Digital caliper 160 (SOMET, Czech Republic) and the values were recorded in mm units. The measurements were applied for 10 tablets and the mean values were recorded. Tablet thickness should be controlled within ± 5% variation of the mean value [44].
Uniformity of Weight
The weights of 20 tablets were weighed individually using an analytical balance (Setra BL-410S, China) then the weight of each tablet was compared with the respective average weight of the tablets. The tablets met the test if not more than two tablets deviated from the average weight by more than 5% [44].
Friability Test
Twenty tablets were accurately weighed (W1) and placed in the drum of the friability tester (Pharmatest PTF 10E, Germany) where they were subjected to rolling and repeated shocks by apparatus’ septa that present inside the transparent drum. After four minutes of this treatment at a speed of 25 rpm (100 revolutions), the tablets were removed from the drum, carefully brushed to remove adhering dust, and then re-weighed (W2). The percentage loss in weight due to abrasion was the measured parameter for assessment of tablet friability and was calculated from the following equation:
The % loss should not be more than 1.0% of the weight of the tablets that being tested [44].
Hardness Test
The crushing strength (hardness) of ten tablets was determined using hardness tester (AHIBA CH4127 Birsfelden, Type TPG20) and the values were recorded in (kg/cm2) units. The mean value of ten measurements was calculated. The prepared tablets met the requirements if all tablets fall in the acceptable range of hardness (from 4.9 to 6.8 kg/cm2) [45, 46].
Drug Content
This test was done by testing the drug content for ten tablets of the selected formula (F4). Each tablet was crushed individually and extracted in 20 ml methanol, then 1 ml was taken and further diluted to 4 ml with methanol then further diluted by the same volumes three times more. The solution was filtered through a micro pore sterile syringe filter (0.45 µm, Pall Corporation, Michigan, USA) and the absorption was recorded by UV spectroscopy at λ max 260 nm.
The requirements for content uniformity are met if not more than one individual content is outside the limits of 85 to 115% of the average content and none is outside the limits of 75 to 125% of the average content [44].
In Vivo X-ray Studies on Dogs for the Selected (F4) Tablets
In vivo X-ray studies were carried out on dogs to assess the in vivo performance of the selected compression-coated tablet formulation (F4). The main purpose of this study was to monitor the movement, integrity, and disintegration of the tablet throughout the gastrointestinal tract of dogs. These studies were carried out at Surgery, Anesthesiology and Radiology department, Faculty of Veterinary medicine, Cairo University. The proposal of this experiment was approved by the Animal Ethics Committee of Faculty of Pharmacy, Helwan University, no. 15A2021. The core tablet formula was prepared using the same amounts of the original ingredients of F4 except that the amount of rutin was replaced with 50 mg barium sulfate as a contrasting agent (X-ray opaque material). The prepared core tablets were subjected to further coating with 220 mg of coating materials (same to the selected formula F4) to get a total 350 mg compression-coated barium sulfate tablets using the same compression force as before. The experiment was conducted on three mongrel dogs of 1–2 years old and 15–20-kg body weight. The experimental animals were fasted overnight before the administration of the tested formula. In the next morning, the dogs were radiographed before administration of the tested tablets for exclusion of radiographic abnormalities and radiopacity confusion. Then, the dogs administered the tablets orally and radiographed, just after administration and then at 1-, 2-, 3-, 4-, 6-, 7-, 8-, and 10-h time intervals. The dogs were X-ray examined at lateral position using Fischer X-ray machine (Model (EMERALD-125), EUREKA TUBE CO., Chicago, IL, USA).The X-ray films were photographed using digital camera.
In vitro Cytotoxicity Against Human Colon Carcinoma Cell Lines Using Sulforhodamine B Assay
The in vitro cytotoxicity of pure rutin, rutin-PVP K30 solid dispersion, frankincense, and a mixture of rutin SD and frankincense in a ratio representing their concentrations in the compression-coated tablet (F4) was assessed against human colon cancer (HCT-116) cell lines using sulforhodamine-B (SRB) assay.
Human Tumor Cell Lines
Human colon carcinoma cell lines (HCT-116) used in this study were obtained from the American Type Culture Collection (ATCC, USA). The tumor cell lines were maintained by serial sub-culturing at the cancer cell line special unit in National Cancer Institute, Cairo, Egypt, The cell lines were grown in Roswell Park Memorial Institute-1640 medium supplemented with 10% heat-deactivated fetal bovine serum, 2 mM l-glutamine, and 50 µg/mL gentamicin in a 37°C humidified incubator and 5% CO2 atmosphere. Cell viability was determined at the beginning of the experiment by trypan blue dye exclusion method [47]. Cell viability was more than 98%.
Samples Preparation
Samples were prepared by being dissolved in dimethyl sulfoxide and stored frozen at − 20°C prior to use. The potential cytotoxicity of different samples was tested using SRB assay according to a method reported previously [48]. SRB is a bright pink aminoxanthrene dye with two sulfonic groups. It is a protein stain that binds to the amino groups of intracellular proteins under mild acidic conditions to provide a sensitive index of cellular protein content. The SRB colorimetric assay was used for cytotoxicity screening according to the protocol of National Cancer Institute (Nat. Protoc.2006: 1, 1112–1116). Human colon cancer (HCT-116) cells were seeded in 96-well microtiter plates at a concentration of 4 × 103 cells per well in a 200 μl fresh culture medium and incubated for 24 h in 5% CO2 atmosphere at 37°C to attach to the plates. Then, cells were incubated with concentrations (0, 31.25, 62.5, 125, and 250 μg/ml) of either pure rutin, rutin-PVP K30 SD, frankincense, or the mixture of rutin-PVP K30 SD with frankincense. Three wells were prepared for each individual concentration and the plates were incubated for 48 h at 37°C and in atmosphere of 5% CO2. Following 48-h treatment, the cells were fixed in situ by gentle addition of 10 μl cold 50% (w/v) trichloroacetic acid (TCA) at 10% final concentration TCA and incubated for 1 h at 4°C. The supernatant was discarded and plates were washed 5 times with distilled water using (automatic washer Tecan, Germany) and then dried in air. The plates were stained by addition of 50 μL 0.4% (w/v) SRB solution dissolved in 1% acetic acid to each of the wells and then the plates were incubated for 30 min in dark at room temperature. After staining, unbound stain was removed by washing the plates with 1% acetic acid and air-dried. Then the bound stain was then solubilized with 200 μl of 10 mMtris base (pH 10.5) per well. Tris base was prepared by dissolving 121.1 gm of tris base in 1000 ml distilled water and pH was adjusted by 2 M HCl. Finally, the optical density of each well was measured spectrophotometrically at 570 nm with an ELISA microplate reader (Sunrise Tecan READER, Germany). The percentage of cell survival was calculated as follows:
The concentration of drug required to produce 50% inhibition of cell growth (IC50) was also calculated using Graph Pad Prism 5 ®Statistical software (San Diego, CA, USA) [48].
Statistical Analysis
Statistical analysis of the in vitro cytotoxicity experiment data was accomplished using two-way ANOVA multiple comparisons (Graph Pad Prism 5 ®, San Diego, CA, USA), followed by Tukey’s multiple comparisons test. Data were expressed as mean ± standard deviation. A statistically significant difference was considered at p value < 0.05.
Investigation of Cytomorphological Changes Using Inverted Microscope
Human colon cancer (HCT-116) cells were seeded in 6-well microtiter plates at a concentration of 10 × 103 cell per well in 3 ml fresh culture medium and incubated for 24 h in 5% CO2 atmosphere at 37°C to attach to the plates. Then cells were incubated with 0, 24.5, and 250 μg/ml of the mixture of rutin-PVP K30 SD with frankincense in the same ratio of the selected F4-coated tablet. The zero μg/ml concentration was a control while the concentrations (24.5 and 250 μg/ml) were the IC50 and the maximum treatment concentration, respectively. Two wells were prepared for each individual concentration and the plates were incubated for 48 h at 37°C under atmosphere of 5% CO2. Then, the cytomorphological changes in the incubated cells were visualized and photographed by inverted light microscope from Leica Microsystems (Leica DM IL LED, Germany) at 10 × magnification power.
Stability Studies for the Selected Compression-Coated Tablets (F4)
The aim of stability studies is to ensure how the quality of the prepared tablets varies as function of environmental factors such as temperature and humidity [49].
The selected compression-coated tablets (F4) were packed in an amber glass bottle which was then inserted into a larger sealed glass container filled with saturated solution of sodium chloride to maintain a particular value (75%) of relative humidity (RH) around samples upon storing in a thermostatically controlled incubator chamber (FTC 90E, VELP scientific, Italy) at 40°C. Samples were kept at 40°C/75% RH for a period of 6 months according to ICH guidelines for accelerated stability studies [50].
Stability of tablets was investigated after storage for 1, 2, 3, and 6 months in terms of physical appearance, hardness, and drug content. The content of rutin in the stored tablets was determined using a validated HPLC assay with slight modifications [51]. The details of HPLC analysis of rutin were described in supplementary material (Online Resource1).
Moreover, the in vitro drug release profile for three tablets from the batch stored for 6 months was compared with that for the freshly prepared tablets through calculation of difference factor (ƒ1) and similarity factor (ƒ2) from Eqss (10) and (11) respectively[52].
where ƒ1 estimates the percent difference between the two curves at each time point and measures the relative error between the two curves, \(n\) is the number of time points, Rt is the dissolution value of the reference (freshly prepared tablets) at time t, and Tt is the dissolution value of the test at time t. ƒ1 value of less than 15.0 and ƒ2 value of more than 50 show that the two profiles are similar.
Results and Discussion
Determination of Saturation Solubility of Pure Rutin and the Prepared SDs of Rutin
The saturation solubility (mg/ml) of pure rutin, rutin-skimmed milk SD, and rutin-PVP K30 SD in 0.1N HCl (pH1.2) was 0.143 ± 0.02, 0.145 ± 0.06, and 0.208 ± 0.02, respectively, while the saturation solubility (mg/ml) of pure rutin, rutin-skimmed milk SD, and rutin-PVP K30 SD in phosphate buffer (pH7.4) was 0.106 ± 0.03, 0.202 ± 0.06, and 0.345 ± 0.04, respectively. Thus, rutin-PVP K30 SD (1:1 w/w) achieved higher saturation solubility compared to rutin-skimmed milk SD (1:1 w/w), especially in phosphate buffer pH 7.4 which is a simulated medium for colonic fluids and a target site for rutin release in this study. Thus, rutin:PVP K30 SD was selected to be incorporated into the core of rutin colon-targeted tablets.
Determination of Swelling Percent of Frankincense Powder
The results showed that there was no change in the weight of frankincense powder after swelling test (w2 = w1 = 1 g) indicating that frankincense powder had no swelling ability upon contact with 0.1 N HCl or phosphate buffer pH 7.4. This could be attributed to the hydrophobic nature of frankincense powder which prevented its possible hydration by the swelling medium.
Differential Scanning Calorimetry
Figure 1 illustrates the DSC thermograms of pure rutin, PVP K30, rutin-PVP K30 SD, SSG, Avicel® PH 101, frankincense, gelatin, and the physical mixture of equal weights of rutin/PVP K30 SD and all the used ingredients.
The DSC thermogram of rutin showed three characteristic endothermic peaks at 121.71, 167.45, and 179.23°C. The endothermic peaks at 121.71 and 167.45°C could be attributed to the consecutive stages of dehydration of crystalline water [53, 54].
The endothermic peak at179.23°C was corresponding to the melting point of rutin [20].
The disappearance of rutin endothermic peaks in the thermogram of rutin-PVP K30 SD rutin could be attributed to the formation of amorphous solid dispersion as rutin was molecularly dispersed in PVP K30 [55].
Fourier Transform Infrared Spectroscopy
Figure 2 shows the FT-IR spectra for pure rutin, PVP K30, rutin-PVP K30 SD, SSG, Avicel® PH 101, frankincense, gelatin, and the physical mixture of equal weights of rutin/PVP K30 SD and all the used ingredients. The FT-IR spectrum of rutin showed characteristic peaks at 3417.86 cm−1(OH stretching), 2986.81 cm−1(C–H stretching), 1668.78 cm−1 (C = O stretching), and aromatic structure [56].
The spectrum of the total physical mixture of rutin drug with overall ingredients recorded peaks at 3417.86 cm−1, 2985.66 cm−1, and 1668.78 cm−1. Thus, there were no considerable changes in the FT-IR peaks of total physical mixture of rutin drug with overall ingredients in comparison with the pure drug FT-IR peaks. This indicated that there was no chemical interaction between rutin and the other used excipients.
In Vitro Release Studies of Rutin from the Prepared Tablets
Figure 3 showed in vitro release profiles of rutin from the prepared five formulae of tablets (F1–F5). The results indicated that F1 tablets in which the coating material composed of 100% frankincense showed no release of the drug and the tablets remained intact up to 24 h. This might be due to the hydrophobic nature and the strong binding properties of frankincense that led to formation of highly impermeable coat able to prevent drug release. Therefore, gelatin was incorporated in coat composition with frankincense to act as a channeling agent which by its dissolution, pores are formed in the coat layer facilitating rutin release throughout the impermeable frankincense coating materials [57, 58].
Gelatin was used at four different concentrations (15%, 25%, 35%, and 45% weight per total coat weight) in the tablets’ formulae (F2, F3, F4, and F5), respectively.
Since a lag time of 5 h is sufficient for targeted colonic release [45, 59], the potential of formulae to provide a targeted colonic drug release was evaluated based on the cumulative percentage of rutin released at 5 h. Moreover, most drug dose included in the formulae must be released after reaching the targeted release site. Thus, the formulae were compared also based on the cumulative percentage of rutin released at 24 h. The % rutin released after 5 h was 2.75 ± 0.28%, 6.18 ± 0.97%, 8.42 ± 0.80%, and 25.73 ± 5.82% for F2, F3, F4, and F5 respectively, while at 24 h, the % rutin released from F2, F3, F4, and F5 was 41.04 ± 5.23%, 56.81 ± 5.55%, 82.05 ± 1.78%, and 84.68 ± 1.45%, respectively. From release data, it could be observed that the drug release increased with increasing gelatin content in the coat layer. This was due to increased porosity in the tablet coat as result of gelatin dissolution. Among the tested formulae, both tablets F4 and F5 revealed highest % rutin released at 24 h. There was no significant difference (p ˃ 0.05) between % rutin released at 24 h from F4 and F5. However, F4 provided significantly ( p < 0.05) less % drug released compared to F5 at 5 h. Thus, the formula F4 was selected as a promising device for colon targeted delivery of rutin.
In Vitro Release Kinetics and Mechanisms
The data of rutin release from the prepared coated tablets (F2, F3, F4, and F5) were fitted to zero order and first order as well as Higuchi’s models. As indicated from Table II, the formula F2 followed zero-order kinetics while all the other formulae followed Higuchi kinetics. Moreover, fitting the obtained data of the drug release to Korsmeyer-Peppas model showed n values for all formulae higher than 0.89, indicating that the release mechanism is super case II release. Super case II release involves the simultaneous occurrence of diffusion, polymer relaxation (due to reduced attractive forces between polymer chains and increased chains mobility), and erosion (due to dissolution of gelatin) [20, 60, 61].
Characterization of the Selected (F4) Tablets
The results of flow properties in terms of angle of repose, bulk density, tapped density, compressibility index, and Hausner ratio of the powders of core and coating materials of the selected (F4) compression-coated tablets are shown in Table III.The results revealed the acceptable flow properties of core and coating materials.
The prepared tablets showed mean thickness of 3.06 ± 0.126 mm and all the tablets were within the accepted limit. The mean weight of the prepared tablets was 350.40 ± 1.67 mg, so the accepted range for weight was 332.88–367.92 mg and all the tablets met the requirements concerning the uniformity of weight. The friability (%) was found to be 0.54% which was within the accepted limit. The hardness value was found to be 5.6 ± 0.325 kg/cm2, and all the prepared tablets met the requirements of acceptable range of hardness.
The average drug content in the tested 10 tablets was 99.90 ± 2.30% and the content ranged from 96.72 to 104.90%. Thus, the drug content in each of the 10 tablets was within the range of 85–115%, reflecting a high degree of drug content uniformity of the prepared tablets.
In Vivo X-ray Studies on Dogs for the Selected (F4) Tablets
The compression-coated tablets containing barium sulfate were taken by the dogs and X-ray images were captured at different time intervals (0, 1, 2, 3, 4, 6, 7, 8, and 10 h).The three dogs showed the same results. The X-ray images of one of the three dogs are shown in Fig. 4. It was observed that the tablets’ cores containing barium sulfate remained intact in the stomach for 2 h. Also, the cores remained intact in the small intestine for the following 3 h but once it reached the colon, a little change appeared in shape or size of cores after 6 h and 7 h. This could be attributed to the beginning of barium sulfate release from cores in the colon due to dissolution of gelatin and creation of channels in the hydrophobic frankincense coat. Then, a marked reduction in tablet size appeared at 8 h post administration indicating the increased release of barium sulfate from the tablets’ cores. Complete disintegration of cores as very fine discrete opaque particles was clearly observed in images taken at 10 h post administration. Based on these results, the coat of formula F4 provided an efficient protection for the cores in the stomach and the small intestine until reaching the colon.
X-ray images of F4 tablet and its distribution through gastrointestinal tract to one mongrel dog at fixed time intervals (0 h, pre-administration; 1 h, in the terminal of stomach; 2 h, in the beginning of small intestine (especially in the duodenum); 3 h, in the jejunum; 4 h, in distal small intestine (especially in the ileum); 6 h, in the beginning of large intestine; 7 h, 8 h, and 10 h, in the colon)
In Vitro Cytotoxicity Against Human Colon Carcinoma Cell Lines (HCT-116) Using Sulforhodamine-B Assay
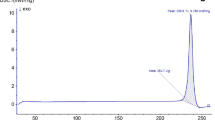
Figures 5 and 6 show the cytotoxic activity of pure rutin, rutin-PVP K30 SD (1:1 w/w), frankincense, and a mixture of rutin-PVP K30 SD with frankincense in a ratio representing their concentrations in the compression-coated tablet (F4) using SRB assay. After 48-h incubation of different concentrations of tested samples with human colon cancer HCT-116 cell line, the results demonstrated a dose-dependent anticancer activity against HCT-116 cell line in all treated cells. Rutin-PVP K30 SD was more effective in arresting cell growth than pure rutin. The IC50 of rutin-PVP K30 SD was 28.5 μg/ml while the IC50 of pure rutin was 31.25 μg/ml. The 8.8% decrease in IC50 of rutin after its incorporation in solid dispersion with PVP K30 could be attributed to the presence of rutin in solid dispersion as amorphous molecularly dispersed form which enables higher cellular uptake.
The cytotoxic activity of pure rutin, rutin- PVP K30 SD (1:1 w/w), frankincense, and a mixture of rutin-PVP K30 SD with frankincense in a ratio representing their concentrations in the compression-coated tablet (F4) against human colon cancer (HCT-116) cell line as revealed from SRB assay. Each point represents the mean of data ± standard deviation (n = 3)
The cytotoxic activity of pure rutin, rutin-PVP K30 SD (1:1 w/w), frankincense, and a mixture of rutin-PVP K30 SD with frankincense in a ratio representing their concentrations in the compression-coated tablet (F4) against human colon cancer (HCT-116) cell line as revealed from SRB assay. Each point represents the mean of data (n = 3). Each column represents the mean ± standard deviation (n = 3). $ represents significance (p < 0.05) of rutin-PVP K30 SD/frankincense mixture versus rutin, rutin-PVP K30 SD, and frankincense at the same concentrations. # represents significance (p ˂0.05) of rutin-PVP K30 SD/frankincense mixture versus both rutin and rutin-PVP K30 SD only. * represents significance (p < 0.05) of rutin-PVP K30 SD/frankincense mixture versus frankincense only
The cytotoxic outcomes of frankincense on HCT-116 cell line showed growth inhibitory effect with IC50 of 42.5 μg/ml, which demonstrates that frankincense has an antitumor activity against colon cancer. The antitumor activity of frankincense resin could be attributed to its chemical composition that contains boswellic acids which have the ability to modulate important biological activities. In a previous study by Liu et al., it was found that boswellic acids possessed anti-proliferative and apoptotic effects on HT29 colon cancer cells [33].
As illustrated in Fig. 6, the rutin-PVP K30 SD/frankincense mixture showed a significant (p < 0.05) higher cytotoxic activity against HCT-116 cell line compared to the individual cytotoxic activity of either pure rutin, rutin-PVP K30 SD, or frankincense at concentrations of 31.25 and 62.50 μg/ml. At 250 μg/ml, the cytotoxic activity of rutin-PVP K30 SD/frankincense mixture was significantly (p < 0.05) higher than frankincense while it was not significantly (p > 0.05) different from rutin or rutin-PVP K30 SD. At 125 µg/ml, the cytotoxic activity of rutin-PVP K30 SD/frankincense mixture was significantly (p < 0.05) higher than that of rutin or rutin-PVP K30 SD while it was not significantly (p > 0.05) different from frankincense.
The IC50 of rutin-PVP K30 SD/frankincense mixture was found to be 24.5 μg/ml. Thus, the presence of frankincense accounted for 21.60% reduction in the IC50 value of rutin-PVP K30 SD/frankincense combination compared to pure rutin and 14.04% reduction in the IC50 value of rutin-PVP K30 SD/frankincense mixture compared to rutin-PVP K30 SD alone.
Thus, these results revealed that a combination of rutin-PVP K30 SD with frankincense provided a successful synergistic effect that could have a higher antitumor activity than pure rutin against human colon cancer.
Investigation of Cytomorphological Changes Using Inverted Microscope
The cytomorphological changes in HCT-116 cells after 48-h incubation with 0, 24.5, and 250 μg/ml rutin-PVPK30 SD/frankincense combination in a ratio representing their concentrations in the compression-coated tablet (F4) were investigated under inverted microscope. Figure 7a shows strongly adherent control malignant cells in form of multiple irregular confluences of polygonal-shaped cells with appearance of few rounded-shaped cells due to cellular polymorphism. In Fig. 7b, the cells treated with 24.5 μg/ml (IC50 concentration) showed reduced confluence with losing their defined morphology and appearance of some dead cells that are poorly adhered. In Fig. 7c, the cells treated with maximum concentration (250 μg/ml) appeared as dead, detached cells with loss of polygonal shape.
The changes observed in the morphology of HCT-116 cells illustrated the cytotoxic effect of rutin-PVPK30 SD/frankincense combination and confirmed the results obtained from in vitro cytotoxicity against HCT-116 using SRB assay.
Stability Studies for the Selected Compression-Coated Tablets (F4)
Accelerated stability studies were carried out for duration of 6 months as per ICH guidelines. As shown in (Table IV), the results revealed that tablets were stable regarding physical appearance, drug content, and hardness after a study period of 6 months. Moreover, the calculated difference factor (ƒ1) and similarity factor (ƒ2) for comparing the release profile of tablets stored for 6 months that of freshly prepared tablets were 7.54 and 76.74, respectively. Therefore, there was no significant difference in the release profile of rutin from the prepared (F4) tablets over the course of 6 months. The overlay of the in vitro release profiles relevant to the freshly prepared F4 tablets and F4 tablets stored for 6 months is illustrated in Fig. 8.
Conclusion
The study revealed that tablets consisting of rutin-PVP K30 SD in the core and coated with a mixture of 65% frankincense and 35% gelatin could provide enhanced rutin solubility with a successful targeted delivery of rutin to the colon. Moreover, the combination of rutin-PVP K30 SD with frankincense enhanced the in vitro cytotoxicity of rutin against human colon cancer (HCT-116) cell line.
Thus, improving rutin solubility using solid dispersion technique along with employing frankincense as a drug release controller with cytotoxic activity against human colon cancer cells could be considered a promising innovative approach for formulation of rutin tablets targeted for colon cancer treatment.
Data Availability
Data will be available upon request.
References
Yuan L, Zhang S, Li H, Yang F, Mushtaq N, Ullah S, Shi Y, An C, Xu J. The influence of gut microbiota dysbiosis to the efficacy of 5-fluorouracil treatment on colorectal cancer. Biomed Pharmacother. 2018;108:184–93.
Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14(10): 101174.
Nouri Z, Fakhri S, Nouri K, Wallace CE, Farzaei MH, Bishayee A. Targeting multiple signaling pathways in cancer: the rutin therapeutic approach. Cancers. 2020;12(8):2276.
Chen S, Gong J, Liu F, Mohammed U. Naturally occurring polyphenolic antioxidants modulate IgE-mediated mast cell activation. Immunology. 2000;100(4):471–80.
Aherne SA, O’Brien NM. Protection by the flavonoids myricetin, quercetin, and rutin against hydrogen peroxide-induced DNA damage in Caco-2 and Hep G2 cells. Nutr Cancer. 1999;34(2):160–6.
Alonso-Castro AJ, Domínguez F, García-Carrancá A. Rutin exerts antitumor effects on nude mice bearing SW480 tumor. Arch Med Res. 2013;44(5):346–51.
Volková M, Forstová-Krízová V, Skálová L, Trejtnar F. Modulatory effects of quercetin and rutin on the activity, expression and inducibility of CYP1A1 in intestinal HCT-8 cells. Phytother Res. 2013;27(12):1889–93.
Jantrawut P, Akazawa H, Ruksiriwanich W. Anti-cancer activity of rutin encapsulated in low methoxyl pectin beads. Int J Pharm Pharm Sci. 2014;6(3):199–202.
Jayameena P, Sivakumari K, Ashok K, Rajesh S. Rutin: a potential anticancer drug against human colon cancer (Hct116) cells. Int J Biol Pharm Allied Sci. 2018;7(9):1731–45.
Nasrabadi PN, Zareian S, Nayeri Z, Salmanipour R, Parsafar S, Gharib E, Aghdaei HA, Zali MR. A detailed image of rutin underlying intracellular signaling pathways in human SW480 colorectal cancer cells based on miRNAs-lncRNAs-mRNAs-TFs interactions. J Cell Physiol. 2019;234(9):15570–80.
Al-Yasiry ARM, Kiczorowska B. Frankincense - therapeutic properties. Postepy Hig Med Dosw. 2016;70:380–91.
Manach C, Morand C, Demigné C, Texier O, Régérat F, Rémésy C. Bioavailability of rutin and quercetin in rats. FEBS Lett. 1997;409(1):12–6.
Mauludin R, Müller RH, Keck CM. Development of an oral rutin nanocrystal formulation. Int J Pharm. 2009;370(1–2):202–9.
Kamel R, Basha M. Preparation and in vitro evaluation of rutin nanostructured liquisolid delivery system. Bull Fac Pharmacy, Cairo Univ. 2013;51(2):261–72.
Vu HTH, Hook SM, Siqueira SD, Müllertz A, Rades T, McDowell A. Are phytosomes a superior nanodelivery system for the antioxidant rutin? Int J Pharm. 2018;548(1):82–91.
Hoai TT, Yen PT, Bich Dao TT, Long LH, Anh DX, Minh LH, Anh BQ, Thuong NT. Evaluation of the cytotoxic effect of rutin prenanoemulsion in lung and colon cancer cell lines. J Nanomater. 2020;2020.
De Gaetano F, Cristiano MC, Venuti V, Crupi V, Majolino D, Paladini G, et al. Rutin-loaded solid lipid nanoparticles: characterization and in vitro evaluation. Molecules. 2021;26(4):1–16.
Wu H, Su M, Jin H, Li X, Wang P, Chen J, Chen J. Rutin-loaded silver nanoparticles with antithrombotic function. Front Bioeng Biotechnol. 2020;8:1–11.
Kızılbey K. Optimization of rutin-loaded PLGA nanoparticles synthesized by single-emulsion solvent evaporation method. ACS Omega. 2019;4(1):555–62.
Asfour MH, Mohsen AM. Formulation and evaluation of pH-sensitive rutin nanospheres against colon carcinoma using HCT-116 cell line. J Adv Res. 2018;9:17–26.
Sri KV, Kondaiah A, Ratna JV, Annapurna A. Preparation and characterization of quercetin and rutin cyclodextrin inclusion complexes. Drug Dev Ind Pharm. 2007;33(3):245–53.
Nguyen TA, Liu B, Zhao J, Thomas DS, Hook JM. An investigation into the supramolecular structure, solubility, stability and antioxidant activity of rutin/cyclodextrin inclusion complex. Food Chem. 2013;136(1):186–92.
Paczkowska M, Mizera M, Piotrowska H, Szymanowska-Powałowska D, Lewandowska K, Goscianska J, Pietrzak R, Bednarski W, Majka Z, Cielecka-Piontek J. Complex of rutin with β-cyclodextrin as potential delivery system. PLoS ONE. 2015;10(3): e0120858.
Koval’skii I V., Krasnyuk II, Krasnyuk II, Nikulina OI, Belyatskaya A V Kharitonov YY, et al. Studies of the solubility of rutin from solid dispersions. Pharm Chem J. 2014;47(11):612–5.
Moin A, Gangadharappa HV, Adnan M, Rizvi SM, Ashraf SA, Patel M, et al. Modulation of drug release from natural polymer matrices by response surface methodology: in vitro and in vivo evaluation. Drug Des Devel Ther. 2020;14:5325–36.
Hamidpour R. Frankincense (Boswellia Species): The novel phytotherapy for drug targeting in cancer. Arch Cancer Res. 2016;4(1):1–5.
Huang K, Chen Y, Liang K, Xu X, Jiang J, Liu M, et al. Review of the chemical composition, pharmacological effects, pharmacokinetics, and quality control of Boswellia carterii. Evid-Based Complement Altern Med. 2022;2022.
Rijkers T, Ogbazghi W, Wessel M, Bongers F. The effect of tapping for frankincense on sexual reproduction in Boswellia papyrifera. J Appl Ecol. 2006;43:1188–95.
Frank MB, Yang Q, Osban J, Azzarello JT, Saban MR, Saban R, et al. Frankincense oil derived from Boswellia carteri induces tumor cell specific cytotoxicity. BMC Complement Altern Med. 2009;9.
Chowdary KPR, Reddy GR. Formulation and evaluation of aceclofenac controlled release tablets employing olibanum resin. Res J Pharm Biol Chem Sci. 2012;3(1):715–24.
Rao B, Shivalingam M, Chowdary K, Sunitha N, Rao V. Preparation and evaluation for controlled release of olibanum resin coated microcapsules of carbamazepine. Int J Pharm Biomed Res. 2011;3.
Yadav VR, Prasad S, Sung B, Gelovani JG, Guha S, Krishnan S, et al. Boswellic acid inhibits growth and metastasis of human colorectal cancer in orthotopic mouse model by downregulating inflammatory, proliferative, invasive and angiogenic biomarkers. Int J Cancer. 2012;130(9):2176–84.
Liu JJ, Nilsson Å, Oredsson S, Badmaev V, Zhao WZ, Duan RD. Boswellic acids trigger apoptosis via a pathway dependent on caspase-8 activation but independent on Fas/Fas ligand interaction in colon cancer HT-29 cells. Carcinogenesis. 2002;23(12):2087–93.
Takahashi M, Sung B, Shen Y, Hur K, Link A, Boland CR, et al. Boswellic acid exerts antitumor effects in colorectal cancer cells by modulating expression of the let-7 and miR-200 microRNA family. Carcinogenesis. 2012;33(12):2441–9.
Rasenack N, Hartenhauer H, Müller BW. Microcrystals for dissolution rate enhancement of poorly water-soluble drugs. Int J Pharm. 2003;254(2):137–45.
Farazin A, Mohammadimehr M, Ghasemi A, Naeimi H. Design, preparation, and characterization of CS/PVA/SA hydrogels modified with mesoporous Ag2O/SiO2 and curcumin nanoparticles for green, biocompatible, and antibacterial biopolymer film. RSC Adv. 2021;11(52):32775–91.
Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci Off J Eur Fed Pharm Sci. 2001;13(2):123–33.
Pillay V, Fassihi R. Evaluation and comparison of dissolution data derived from different modified release dosage forms: an alternative method. J Control release Off J Control Release Soc. 1998;55(1):45–55.
Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15(1):25–35.
Ritger PL, Peppas NA. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J Control Release. 1987;5(1):37–42.
Siepmann J, Peppas NA. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv Drug Deliv Rev. 2001;48(2):139–57.
Carr RL. Evaluating flow properties of solids. Chem Eng. 1965;18:163–8.
The United States Pharmacopeia, US Pharmacopial Convention, MD, USA. 2015. p. 420–4.
Haturvedi Hitesh, Garg Ayush, Rathore US. Post-compression evaluation parameters for tablets-an overview. Eur J Pharm Med Res. 2017;4(11):526–30.
Hashem FM, Shaker DS, Nasr M, Saad IE, Ragaey R. Guar gum and hydroxy propyl methylcellulose compressed coated tablets for colonic drug delivery: in vitro and in vivo evaluation in healthy human volunteers. Drug Discov Ther. 2011;5(2):90–5.
Reddy V, Syed M, Rao DS. Formulation and evaluation of colon targeted oral drug delivery system for meloxicam. 2015;4(1):1–9.
Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol. 2001;Appendix 3:Appendix 3B.
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82(13):1107–12.
Oliveira PR, Mendes C, Klein L, Sangoi M da S, Bernardi LS, Silva MAS. Formulation development and stability studies of norfloxacin extended-release matrix tablets. Biomed Res Int. 2013;2013:716736.
Bajaj S, Singla D, Sakhuja N. Stability testing of pharmaceutical products. J Appl Pharm Sci. 2012;2(3):129–38.
Fang QN, Wu P, Yang L. Separation and determination of puerarin by high performance liquid chromatography. Acta Pharm Sin. 2012;17(9):695–9.
Shah VP, Lesko LJ, Fan J, Fleischer N, Handerson J, Malinowski H, et al. FDA guidance for industry 1 dissolution testing of immediate release solid oral dosage forms. Dissolution Technol. 1997;4(4):15–22.
Horosanskaia E, Nguyen TM, Vu TD, Seidel-Morgenstern A, Lorenz H. Crystallization-based isolation of pure rutin from herbal extract of Sophora japonica L. Org Process Res Dev. 2017;21:1769–78.
Neto CMS, Lima FC, Morais RP, de Andrade LRM, de Lima R, Chaud M V, et al. Rutin-functionalized multi-walled carbon nanotubes: molecular docking, physicochemistry and cytotoxicity in fibroblasts. Toxics. 2021;9(8).
Dedroog S, Pas T, Vergauwen B, Huygens C, Van den Mooter G. Solid-state analysis of amorphous solid dispersions: why DSC and XRPD may not be regarded as stand-alone techniques. J Pharm Biomed Anal. 2020;178: 112937.
Selvaraj KSV, Chowdhury R, Bhattacharjee C. Isolation and structural elucidation of flavonoids from aquatic fern azolla microphylla and evaluation of free radical scavenging activity. Int J Pharm Pharm Sci. 2013;5(3):743–9.
Basher MA, Kalam A, Kabir L. Comparative evaluation of HPMC , PVA and gelatin as matrices for controlled release drug delivery. 2009;51–5.
Saxena A, Tahir A, Kaloti M, Ali J, Bohidar HB. Effect of agar-gelatin compositions on the release of salbutamol tablets. Int J Pharm Investig. 2011;1(2):93–8.
Amidon S, Brown JE, Dave VS. Colon-targeted oral drug delivery systems: design trends and approaches. AAPS PharmSciTech. 2015;16(4):731–41.
Llabot JM, Manzo RH, Allemandi DA. Drug release from carbomer:carbomer sodium salt matrices with potential use as mucoadhesive drug delivery system. Int J Pharm. 2004;276(1–2):59–66.
Al-Taani BM, Tashtoush BM. Effect of microenvironment pH of swellable and erodable buffered matrices on the release characteristics of diclofenac sodium. AAPS PharmSciTech. 2003;4(3):110–5.
Acknowledgements
The authors are grateful to Asst. Prof. Mohammed Said MostafaAmer, Assistant Professor of Surgery, Anesthesiology and Radiology, Faculty of Veterinary Medicine, Cairo University, for his help throughout the experiment of in vivo X-ray studies on dogs.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Aliaa Ismail: conceptualization, data formal analysis, data interpretation, writing—review and editing. Ebtesam El-Biyally: conceptualization, carrying out experiments, data interpretation, and writing—original draft preparation. Wedad Sakran: conceptualization, supervision, and review. All authors have read and approved the manuscript for publication.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ismail, A., El-Biyally, E. & Sakran, W. An Innovative Approach for Formulation of Rutin Tablets Targeted for Colon Cancer Treatment. AAPS PharmSciTech 24, 68 (2023). https://doi.org/10.1208/s12249-023-02518-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02518-7