Abstract
The human eye is a sophisticated organ with distinctive anatomy and physiology that hinders the passage of drugs into targeted ophthalmic sites. Effective topical administration is an interest of scientists for many decades. Their difficult mission is to prolong drug residence time and guarantee an appropriate ocular permeation. Several ocular obstacles oppose effective drug delivery such as precorneal, corneal, and blood-corneal barriers. Routes for ocular delivery include topical, intravitreal, intraocular, juxtascleral, subconjunctival, intracameral, and retrobulbar. More than 95% of marketed products exists in liquid state. However, other products could be in semi-solid (ointments and gels), solid state (powder, insert and lens), or mixed (in situ gel). Nowadays, attractiveness to nanotechnology-based carries is resulted from their capabilities to entrap both hydrophilic and lipophilic drugs, enhance ocular permeability, sustain residence time, improve drug stability, and augment bioavailability. Different in vitro, ex vivo, and in vivo characterization approaches help to predict the outcomes of the constructed nanocarriers. This review aims to clarify anatomy of the eye, various ocular diseases, and obstacles to ocular delivery. Moreover, it studies the advantages and drawbacks of different ocular routes of administration and dosage forms. This review also discusses different nanostructured platforms and their characterization approaches. Strategies to enhance ocular bioavailability are also explained. Finally, recent advances in ocular delivery are described.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eye is a very sensitive organ with a sophisticated physiology. It is composed of anterior and posterior segments. Generally, quality of life is significantly influenced by visual impairment resulted from various diseases. Cataract is the main cause of blindness worldwide. About 40–60% of blindness in the world is caused as a complication of cataract [1]. Early cataract development results from mutations in α, β, and γ crystallin and its associated genes [2]. Glaucoma is a well-known optic neuropathy disease that is connected with elevation in intraocular pressure (IOP). It leads to permanent blindness in the late stage [3]. Furthermore, vision impairment is also related to aging, diabetes, and fungal infection. Examples of ocular diseases include age-related macular degeneration (AMD), diabetic retinopathy (DR), retinoblastoma, and fungal keratitis. A new study valued that approximately 76 million people suffered from glaucoma, 196 million people with AMD, and 92.6 million have DR [1].
Although many potent drugs are available to treat most of ocular complaints, there are many ocular barriers such as tear film, corneal, conjunctival, and blood-ocular barriers that hinder their therapeutic efficacy. Conventional eye drops are wasted by blinking and tear flow. Therefore, their bioavailability is minimized to less than 5% [4]. Cornea is composed of epithelium, stroma, and endothelium. Epithelium allows only the passage of small and lipophilic drug. However, stroma allows the passage of hydrophilic drugs [5]. Endothelium conserves the transparency of the cornea and affords selective entry for hydrophilic drugs and macromolecules into the aqueous humor. The conjunctiva provides a minor impact to drug absorption compared to the cornea, though certain macromolecular nanomedicines, peptides, and oligonucleotides penetrate to the deep layers of the eye absolutely through these tissues. Blood-ocular barriers prevent the passage of xenobiotic compounds into the blood stream. They are classified into blood-aqueous barrier (BAB) in the anterior segment and blood-retinal barrier (BRB) in the posterior segment of the eye [6].
Ocular formulations are intended to be applied on the anterior surface (topical route) of the eye, delivered intraocularly (inside the eye), periocularly (subtenon or juxtascleral), or in combination with ocular devices. Ocular dosage forms could be liquid, semi-solid, solid, or mixed. Liquid dosage include drops, suspension, and emulsion. Eye drops represent more than 95% of the marketed ocular products [7]. They are used for delivering the medication into the anterior part of the eye but with short residence time [5]. Ocular suspensions and emulsions have the ability to deliver hydrophobic drugs, but may lead to blurred vision. Ocular gels and ointments (semi-solid) could significantly enhance residence time. Solid dosage forms could be used to deliver water-sensitive drugs (powder), provide zero order release model (insert), or sustain residence time (therapeutic contact lens) [7].
Effective ocular absorption necessitates appropriate corneal penetration along with effective precorneal residence time, so as to reach and preserve an acceptable drug concentration with the minimum quantity of the active therapeutic constituent. Nanosystems are innovative technologies developed to get through ocular obstacles, shield the drug from the biological environment, sustain drug residence time, and improve corneal permeation across biological barriers [8]. Characterization of the constructed nanosystems is of great importance to ensure its ability to accomplish the required activity. There are many approaches for characterization such as visual appearance, stability, size, zeta potential, possible interactions, pH measurement, and other important ex vivo and in vivo evaluations [6]. This review highlights the gaps in other published reviews regarding the ocular drug delivery. It described comprehensively ocular drug delivery from different points such as the various anatomical features of the eye, different ocular diseases, obstacles to ocular delivery, different routes of ocular administration, classification of dosage forms, numerous nanostructured platforms, characterization approaches, strategies to improve ocular delivery, and future technologies.
Anatomy of the Eye
Human eye is a very sensitive and complex organ. Figure 1 illustrates the anatomy of human eye [6]. Human eye is composed of anterior and posterior chambers. The anterior segment is composed of tear film, cornea, pupil, lens, and ciliary body. The posterior segment is composed of conjunctiva, sclera, choroid, retina, vitreous humor, and optic nerve. The structure and quantity of tears are controlled by orbital glands and epithelial secretions. Cornea is the front portion of the eye that conveys and focuses light into the eye. It is divided into epithelium, stroma, and endothelium. The epithelium is made of five to seven layers of firmly connected cells. Stroma is a water-based compact layer. The endothelium preserves the transparency of the cornea [6]. Iris is the colored portion of the eye which controls the quantity of light penetrating the eye. The dark center opening in the middle of the iris is called pupil. The pupil changes its size according to the available light. Lens is transparent portion that focuses the light into retina. The ciliary body is made of pigmented and non-pigmented ciliary epithelia, a stroma, and ciliary muscles. Capillaries of ciliary body allow communication between anterior and posterior segments [7]. Vitreous humor is a gel-like, clear, avascular connective tissue that exists between the eye lens and the retina. It is made of 99.9% water, hyaluronic acid, ions, and collagen [7]. The conjunctiva is a delicate transparent membrane lining inside the eyelids and shelter the frontal surface of the sclera. It is a mucous membrane that is composed of three layers, an outer epithelium, a substantia propria enclosing nerves, lymphatic and blood vessels, and a submucosa layer linked to the sclera [9]. The sclera is a continuous of cornea. It is made of collagen and mucopolysaccharides. Choroid is vascular layer that is located between retina and sclera. The retina is thin film of tissue composed of neural and glial cells covering the back of the eye. It produces electrical impulses that are delivered through the optic nerve to the brain [7].
Anatomy of the eye [6]
Ocular Diseases
Cataract
Cataract is chief cause of loss of vision worldwide. About 40–60% of blindness in the world is caused as a complication of cataract [1]. As said by the National Programme for Control of Blindness and Visual Impairment, the major reason of avoidable blindness in India is cataract (62.6%) [2]. Cataract could be defined as the development of cloudiness/opacification in the eye lens. The risk factors include exposure to UV light, diabetes, bad nutrition, genetic determinism, and smoking. Cataract could be divided into three types: cortical, nuclear, or posterior subcapsular. The clearness and transparency of lens is regulated by crystallin protein [10]. Alterations in α, β, and γ crystallin and its associated genes are responsible for the early cataract development. Triggers to cataract are glycation, oxidative stress, and exposure to lipophilic compounds which result in increasing calcium level in the lens plus crystallin accumulation. Oxidative stress is mediated by hyperglycemia and hydroxyl radicals. Nowadays, surgical removal of opaque lens is the treatment choice. However, the early employment of anti-cataract agent may minimize surgical treatment. Anti-cataract agents are multifunctional antioxidants with radical hunting and chelation ability [10]. Examples of anti-cataract agents include curcumin, lanosterol, resveratrol, and metformin [11].
Glaucoma
Glaucoma is a famous optic neuropathy disease. Symptoms start with blurred vision that progresses into irreversible blindness in the late stage. It leads to blindness as a result of slow deterioration of optic nerve axon and fatality of retinal ganglion cells [3]. It is commonly connected with elevation in intraocular pressure (IOP) because of irregular formation or obstruction of the aqueous humor [12]. Risk factors include age, race, diabetes, genetics, nearsightedness, migraine, and retinal vascular caliber. Glaucoma is more common in women population as they represent 55% of open angle glaucoma, 70% of angle closure glaucoma, and 59% of all forms of glaucoma in 2010 [1]. Worldwide incidence is estimated at 76 million at 2020 and is expected to elevate to 112 million by 2040 [13]. There are two types of glaucoma: open angle and closed angle. Open angle glaucoma has no symptoms and is characterized by enlarging optic disc cupping and visual field that results in elevated prevention of drainage of aqueous humor through trabecular meshwork. However, closed angle is characterized by the elevated pressure resulted from the blockage of outflow pathways [3]. About 76 million people suffered from glaucoma and the number is expected to reach 112 million by 2040 [14]. Glaucoma developed as a result of oxidative and nitrative processes. There are many antioxidant enzymes in aqueous humor for example superoxide dismutase, catalase, and glutathione peroxidase. Their level is decreased as a result of aging and that leads to elevated IOP. Change the equilibrium between oxidants and antioxidants influence the progression of glaucoma [15]. Anti-glaucoma drugs help to adjust either aqueous humor formation or drainage. Many studies were published to enhance glaucoma treatment [12, 16,17,18]. Abd-Elsalam and ElKasabgy developed topical agomelatine-loaded olaminosomes with remarkable anti-glaucoma activity [12]. Eldeep et al. developed topical proniosomal gel-derived niosomes to improve ocular retention and activity of brimonidine tartrate [18].
Age-Related Macular Degeneration (AMD)
AMD is one of the main causes of loss of vision in developed countries. It is more frequent above the age of 50 years. [3]. About 8.7% of worldwide blindness is initiated by AMD AMD [19]. Nearly 196 million people suffered from AMD at 2020 and the number is expected to reach 288 million by 2040 [20]. It is a multifactorial degenerative complaint involving the posterior segment of the eye. Risk factors include aging, smoking, bad nutrition, high blood pressure, and immobility. There is no remedy for AMD till now, but its progression may be reduced by proper medications [21]. AMD could be divided into two types, dry (atropic or non-exudative) and wet (neovascular or emulative). Irregular angiogenesis (development of new blood vessels) in the retinal epithelium is the main character of AMD and results in drusen (yellow deposits under the retina), atropy, and separation of bruch’s membrane [22]. Many cellular growth factors are increased during angiogenesis due to the irregularities in corresponding metabolic pathways as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (FGF), and epithelial growth factor (EGF). A new approach for treatment of AMD involves juxtascleral injections of anecortave cortisone that showed prolonged release for 6 months in the choroid and retina [23]. Moreover, intravitreal injection of the biodegradable Rho kinase and protein kinase C inhibitor for handling diabetic macular edema and neovascular age-related macular degeneration exhibited prolonged release for about 6 months [24].
Conjunctivitis
Conjunctivitis is generally the most frequent ocular compliant. It is simply the inflammation of conjunctival tissue. It affects all ages, races, and genders [25]. According to the cause, it may classified into infectious or non-infectious. Infectious conjunctivitis results from microbial infection, while non-infectious conjunctivitis results from allergens and irritants [25]. Symptoms of conjunctivitis include eye redness, eye discomfort, tears, and elevated eye secretions. Prevalence of allergic conjunctivitis is nearly 40% of global population [26]. Treatments of conjunctivitis include topical administration of antimicrobial (infectious) or anti-inflammatory agents (non-infectious).
Diabetic Retinopathy (DR)
Diabetic retinopathy is particular vascular complication related to both types of diabetes mellitus. About 60% of patients of type II and all patients of type I diabetes have a certain extent of retinopathy after 20 years of diabetes. Oxidative stress and inflammation result from the upregulation of proinflammatory mediators initiated by hyperglycemic disorders are the cause of development of DR. It is the third major trigger to blindness in the USA. After cataract and corneal blindness, the first and second triggers to blindness. It is avoidable if distinguished and cured early plus the effective management of blood glucose and blood pressure [27]. It has two types, proliferative and non-proliferative and both of them result eventually in progressive damage to the retina. Nowadays, diabetic retinopathy is managed through laser photocoagulation, vitrectomy, and pharmacological treatments. Laser photocoagulation works by closing the leaky blood vessels and possibly avoids blindness, but results in laser scar. Vitrectomy is a surgical removal of vitreous gel and blood from leaking vessels in the back of the eye, but this procedure provides only short-term relief and does not obstruct further leaking of blood [1]. Pharmacological treatments include intravitreous injections of corticosteroids to decrease the swelling of macula. Also, sustained release corticosteroids implant that interferes the inflammatory pathways. Modern management with anti-VEGF agents (Ranibizumab and Aflibercept) prevents the expression of VEGF and thus reduce blood leakage and edema [28].
Retinoblastoma
Retinoblastoma is a malignant tumor distressing the retina and mainly prevails in children younger than 5 years old. Untreated retinoblastoma leads to blindness and finally mortality (99%). Its frequency is about 1 out of 20,000 live births [29]. Its occurrence rate is equal in both gender. It is caused due to mutation in tumor suppressor gene RB1 encoding for retinoblastoma protein. It could be unilateral (60%) or bilateral (40%) [1]. The handling choices of retinoblastoma include radiotherapy, cryotherapy, systemic chemotherapy, and surgery. Latest studies propose that release of compensatory proangiogenic factors and angiogenic blood vessels development are the vital phases for treating retinoblastoma [30].
Fungal Keratitis
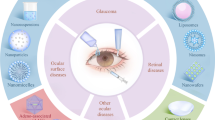
Fungal keratitis occurs only in traumatic cornea, since healthy cornea would not allow any fungal infection. It is caused by different fungus like Candida albicans, Candida glabrata, Candida tropicalis, Candida krusei, and Candida parapsilosis [31]. Fungal keratitis represents 40% of the contagious keratitis in developing countries of third world [1]. Risk factors may be ocular (trauma, contact lens, prior corneal surgery, and topical corticosteroids) or systemic (diabetes, HIV positivity, and leprosy). Fungal keratitis leads to impaired wound healing, corneal ulceration, and stromal inflammatory infiltration. The corneal inflammation may alter miRNA expression [32]. Oral or topical antifungal drugs are used to treat fungal keratitis. Corneal surgery approach could be required when the medicines are useless. In some situations, vision may not be restored even after surgery. Many papers consider the treatment of fungal keratitis. Younes et al. developed topical Sertaconazole nitrate loaded cubosomes and mixed micelles with enhanced safety and antifungal activity [33,34,35,36,37]. Figure 2 illustrates various ocular diseases [1].
Various ocular diseases affecting the two segments of the eye [1]
Obstacles to Ocular Delivery
Precorneal Barriers
Capacity of Cul-de-sac
Figure 3 explains different ocular barriers. Cul-de-sac is a shallow pocket in the lower eyelid where palpebral and bulbar conjunctiva meet in the lower eyelid and the deeper recess in the upper eyelid. The maximum capacity of cul-de-sac is 30 μL in humans. The movement of the lower eyelid to its regular place would reduce this capacity to 70–80%. Inflammation and allergic response of the eye would also minimize capacity of the cul-de-sac [6]. Since the activity of any drug is directly related to its residence time and concentration. The low capacity of cul-del-sac would reduce the concentration of the drug in the eye, thereby reducing the therapeutic activity.
Loss of Drug from Lacrimal Fluid
Drainage of the administrated ocular solution is a major barrier in precorneal region. Loss of drug from lacrimal fluid could happen as a result of lacrimation, solution drainage, and non-productive absorption in the conjunctiva [38]. Also, drug metabolism and protein binding would further obstruct drug absorption [6]. Continuous renewal of lacrimal fluid helps maintaining eye hydration, preventing pathogens or dust from retaining on the eye. In order to maintain effective drug activity, residence time of the administrated formula must be sustained which could be achieved by different mechanisms.
Corneal Barriers
The cornea provides a resistant obstacle to different chemical and mechanical injuries. It also supports light convergence on the retina. It is divided into epithelium, stroma, and endothelium. The epithelium is formed of five to seven layers of firmly linked cells. Stroma is a water-based compact layer. The epithelium is a barrier for hydrophilic drugs and large molecules, whereas the stroma is a barrier for lipophilic drugs [5]. The endothelium preserves the transparency of the cornea and provides selective access for hydrophilic drugs and macromolecules into the aqueous humor. As a general rule, drug molecular weight, charge, degree of ionization, and hydrophobicity influence the corneal permeation. Hence, the trans-corneal permeation is known as rate limiting step for drug transfer from the lachrymal fluid into the aqueous humor [6].
Blood-Ocular Barriers
They prevent the entry of foreign compounds into the blood stream. They are classified into blood-aqueous barrier (BAB) and blood-retinal barrier (BRB). BAB is an anterior portion of the eye that prevents the access of many compounds in the intra-ocular milieu. BAB allows the passage of lipophilic and small drugs. These drugs are eliminated from the anterior compartment faster than hydrophilic and larger molecules. For example, the clearance rate of pilocarpine was found to be faster than inulin [6]. BRB is a posterior portion of the eye that is made of the retinal endothelial cells and the retinal pigment epithelial cells. It prevents the access of dangerous materials, water, and plasma components into the retina [39].
Routes for Ocular Drug Delivery
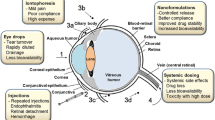
Figure 4 illustrates different routes for ocular drug delivery [7].
Different routes for ocular drug delivery [7]
Topical Administration
Topical administration is the most common route for ocular drug delivery that represents more than 95% of marketed ocular products. It is non-invasive route, but with low bioavailability (<5%) due to insufficient corneal permeation and short residence time [4]. Moreover, bioavailability is reduced by tear drainage, blinking and entering the systemic circulation through the nasolacrimal pathway. Topical delivery necessitates frequent and high dose concentration, which could result in serious side effects. Also, frequent dosing could influence the patient compliance [4, 5]. Topical route is unsuitable for handicapped and elder patients [7]. The topical administration of terconazole in the form of bilosomes showed enhanced drug permeation and safety [40]. Topical administration of sertaconazole nitrate as mixed micelles or cubosomes revealed high corneal uptake and corneal retention [33, 34]. β-cyclodextrin-based micellar system demonstrated higher ex vivo and in vivo permeation of itraconazole and higher antifungal activity [35]. The topical administration of dorzolamide hydrochloride in the form of proniosomal gels showed controlled ex vivo permeation, increased stability, and improved bioavailability [16]. Table I reveals more examples of topical administration.
Intracameral Injections
Intracameral injections involve injection of antibiotic directly into the anterior segment of the eyeball or in the vitreous cavity. It is done usually subsequent to cataract surgery to avoid endophthalmitis initiated by a contagion of the eye that can occur after cataract surgery. Recently, the application of intracameral injection for treatment of glaucoma using hydrogel functionalized with vinyl sulfone and thiol groups was published [73].
Intravitreal Injections/Implants
Intravitreal injection is a delivery of medicine into the vitreous that is close to the retina at the back of the eye. A new approach for treatment of glaucoma includes a single intravitreal injection of vitamin E/poly-lactic-co-glycolic acid microspheres enclosing glial cell line derived neurotrophic factor. This approach provided a prolonged release for 6 months. Similar results was obtained after intravitreal injection of polymer-free dexamethasone dimer implants [52]. Intravitreal injection of the biodegradable Rho kinase and protein kinase C inhibitor for handling diabetic macular edema and neovascular age-related macular degeneration exhibited prolonged release for about 6 months [24]. Additional examples are mentioned in Table I.
Juxtascleral Injections
Juxtascleral injections are used for treatment of some posterior part complaints that cannot be handled through conventional topical route. It is used for the treatment of cystoid macula edema, trauma, and diabetic-related conditions. A new approach for treatment of AMD involves juxtascleral injections of anecortave cortisone that showed prolonged release for 6 months in the choroid and retina [23]. Advanced trans-scleral microneedles have been formulated to carry adeno-associated viruses for retinal gene treatment [55]. Table I shows further examples.
Retrobulbar Injection
Retrobulbar route involves the injection of a needle through the eyelid and orbital fascia to deliver the medication behind the globe into the retrobulbar space. Retrobulbar injection of amphotericin B showed higher antifungal efficacy than intravenous injection [58]. Retrobulbar injection of chlorpromazine is used to manage blind painful eyes [59]. Retrobulbar injection of triamcinolone is utilized to handle macular edema resulted from retinal vein occlusion [60].
Subconjunctival Injection
Subconjunctival injection is frequently used in cases of very low drug penetration into the anterior part of the eye after topical administration. Subconjunctival injection of steroids fabricated as PEGylated liposome for handling of uveitis showed sustained anti-inflammatory activity and targeting the required ocular tissue for 1 month as minimum [61]. The administration of PLGA nanoparticle of brinzolamide by subconjunctival injection showed successful handling of IOP for 10 days [62]. Significant lowering in corneal inflammation and squamous metaplasia was ensured via subconjunctival injection of human mesenchymal stromal cells in mice with graft versus host disease [63]. Table I shows extra examples.
Irrigating Solutions
They are solutions made under aseptic condition without the inclusion of preservatives. They are used as balanced salt by surgeons to eradicate blood, cellular waste, and maintain the appropriate hydration volume of the eye [74]. There are many examples that intensifies the importance of these solutions. For example, minimizing the cataract surgical duration and avoiding pupil miosis by using ketorolac (0.3% w/v) and phenylephrine (1% w/v) in the irrigation solutions [66]. Table I shows more examples.
Iontophoresis
Iontophoresis is a technique used to carry medications into the posterior segment of the eye. It involves the usage of voltage gradient. Novel systems involve the employment of microneedle-based instruments. They had doubled the amount of formula delivered to the back of the eye compared to suprachoroidal injection [75]. The combinations of iontophoretic delivery and contact lens results in 550–1300-times shorter duration than drug uptake into choroidal capillaries [76]. Short-duration iontophoresis of acyclovir prodrug resulted in higher permeation and bioavailability [69]. Ocular iontophoresis of dexamethasone phosphate revealed higher efficacy in managing non-infectious anterior uveitis [70]. Table I shows more examples.
Dosage Forms
Liquid Dosage Forms
Eye Drops
Eye drops represent more than 95% of the marketed ocular products. They deliver the medication into the anterior part of the eye. Their advantages include easy administration and accepted stability. However, their disadvantages include low retention time (<5 min.), poor bioavailability, and serious side effect resulted from the frequent administration of high concentration [77]. Several nanosystem platforms had been developed to solve their drawbacks. Cyclosporine was formulated as a mucoadhesive nanosystem utilizing poly (D-L-lactide)-b-dextran. Nanoprecipitation technique was adopted for the formulation. The final product demonstrated small particle size, enhanced permeability, and drug retention [43]. Formulation of the antibacterial hesperetin as micellar system showed minute particle size, high percentage entrapment efficacy, greater penetration, and enhanced efficacy [44]. More examples are illustrated in Table II. Figure 5 illustrates different ocular dosage forms.
Eye Suspensions
Ocular suspensions represent dispersions of hydrophobic drug in aqueous solvent. They have enhanced contact time because of drug retention in the conjunctival cul-de-sac. Particle size, solubility, and dissolution rate in the tear fluid are extremely important during the preparation process [102]. Generally, particle size <10 µm has greater solubility, enhanced dissolution rates, and poor retention on the ocular surface. However, particles of >10 µm could result in ocular irritation and stimulated tearing [103]. Disadvantages of ocular suspension include poor stability. They cannot be stored in freezer as the particles tend to agglomerate and fail to disperse easily. Also, change in crystal size during the storage will influence both solubility and bioavailability of the drug. A blurred vision after their administration could also result. Improved ocular administration of posaconazole in polymer system of carbopol 974P and xanthan gum using high pressure homogenizing technique showed enhanced stability, antifungal activity, and prolonged retention [46]. High speed liquid–liquid shear technique was adopted to formulate ultra-fine rebamipide ophthalmic suspension. This formula showed enhanced transparency, small particle size, and improved stability [45]. Table II states more examples.
Eye Emulsions
An emulsion is a solubilized biphasic system due to the inclusion of surfactants or stabilizers. Advantages of eye emulsions include ability to deliver hydrophobic drugs; oil-in water (O/W) emulsion is less irritant to the eye, enhanced contact time and bioavailability [104]. The ocular delivery of dexamethasone acetate and polymyxin B sulfate was enhanced by the formation of nanoemulsion by high-pressure homogenization. A positive charge inducer was incorporated to enhance ocular adhesion. The resulted formula showed enhanced stability, reduced particle size, and enhanced retention time [84]. Water titration method was adopted for the construction of triamcinolone acetonide microemulsion. It showed minimized particle size and improved permeability [85]. Additional results are clarified in Table II.
Semisolid Dosage Forms
Eye Gels
Eye gels are a semisolid dosage form containing high water quantity. They have enhanced retention time and bioavailability because of their viscosity. Although gels contain large quantity of water, blurred vision could still result. Various polymers could be used to prepare ocular gels like polyacrylic acid, acrylic acids, hydroxypropyl methylcellulose, and carboxymethyl cellulose [105]. Coacervation technique was used to prepare a proniosomal gel of curcumin with effective reduction in particle size and improvement of anti-inflammatory activity [89]. An increase in the ex vivo permeability and retention time of pilocarpine was demonstrated via formation of phytantriol-based lyotropic liquid crystalline gel. That gel was formed by vortex method [90]. Additional examples are mentioned in Table II.
Eye Ointments
Eye ointments are semisolid dosage form containing white petrolatum and mineral oil. They are administrated to the lower eyelid only at bedtime due to its interference with vision. They are commonly used among young patients. They have anhydrous nature making them a good choice for lipophilic and moisture sensitive drugs. They have higher retention time and bioavailability in comparison with solutions [106]. Avaclyr® is an ocular ointment enclosing the antiviral acyclovir that was approved in 2019 for herpetic keratitis. Also, Lotemax® enclosing the anti-inflammatory loteprednol etabonate. Both of them showed enhanced corneal penetration and drug release [92].
Solid Dosage Forms
Eye Powders
They are sterile solid dosage form of water-sensitive drugs. They are administrated in injectable forms as intracameral injection of cefuroxime, moxifloxacin, and voriconazole. Cefuroxime and moxifloxacin are reconstituted in saline, while voriconazole is reconstituted in water. Both cefuroxime and voriconazole solutions are stable for 7 days after reconstitution. However, moxifloxacin solution is stable for 24 weeks [107, 108].
Ocular Inserts
Ocular inserts are solid dosage form of biodegradable polymers. They show zero order drug release model. Advantages of inserts include high residence time, sustain drug delivery, constant release, and reduced side effects [109]. Electrospinning technique was adopted for the construction of triamcinolone acetonide-loaded nanofibers. They showed reduced particle size, systemic absorption, and side effects [93]. Also, sustained bimatoprost activity for many months was proved after incorporation of its insert [110]. Table II shows more examples.
Therapeutic Contact Lens
New studies showed that therapeutic contact lens could enhance bioavailability by >50% as a consequence to sustained residence time and close contact with the cornea [111]. Their residence time is 10 folds the conventional eye drops [112]. They also reduce required doses, interval between doses and systemic absorption [113]. There are many techniques to enclose the drug inside contact lens as molecular imprinting, ion ligation, soaking, and use of nanoparticles [77, 114, 115]. Obstacles to their clinical use include protein attachment, ion and oxygen permeation, drug loss during manufacture or storage, transmittance, and swelling of the lens [7]. Dexamethasone contact lens was prepared by encapsulation technique. It showed 200-fold drug retention in the retina matching with conventional eye drops [116]. In order to reduce rapid drug release, chips of either timolol, bimatoprost, or hyaluronic acid have been used [111]. Extra examples are given in Table II.
Mixed Dosage Forms
In Situ Gel
They are polymeric solutions of low viscosity. They converted into pseudo-plastic gels in contact with tear fluid. They have sustained contact time compared to simple solutions [117]. There are three types of in situ gel according to the transition properties: temperature, ionic, or pH sensitive [118]. In situ gel of ciprofloxacin with hydroxypropyl methylcellulose and sodium alginate (ion-sensitive) showed enhanced residence time and sustained drug release [98]. Thermosensitive in situ gel of hydrocortisone butyrate revealed extended drug release and avoided burst release [99]. Thermosensitive in situ gel of ketorolac tromethamine showed improved mucoadhesive properties with prolonged release of drug up to 12 h [119]. Table II shows more examples about in situ gel.
Nanostructured Platforms
Liposomes
They were discovered in the mid-1960s [120]. Advantages of liposomes include safety, biodegradation, simple preparation techniques, and improved bioavailability [121]. They are spherical nanocarriers made of one or more concentric lipid bilayers. They could carry lipophilic drug in the lipid area, while the interior could entrap hydrophilic drugs. Changing the formation technique and their composition could alter their surface charge, sensitivity to ion or pH, or temperature changes and the resulted particle size [120]. Generally, the corneal epithelium has a negative charge; therefore, a positively charged liposomes would have high adherence, longer retention time, and better absorption. These outcomes will reduce the interval between doses and improve patient satisfaction [122]. Zhang and Wang created a liposomal system composed of phosphatidylcholine, cholesterol, α-tocopherol, and chitosan. The resulted formula showed high percent entrapment, sustained activity, and enhanced efficacy [47]. Lin et al. used phosphatidylcholine, stearylamine, cholesterol, and hyaluronic acid. The finished product revealed better corneal uptake, high drug targeting, improved percent entrapment, and prolonged penetration [123]. Cheng et al. constructed liposomal system formed of soybean phosphatidylcholine, cholesterol, chitosan, and dicetylphosphate. This formula showed superior corneal permeation and improved activity [124]. Vicario-de-la-Torre et al. used phosphatidylcholine, cholesterol, sodium hyaluronate, trehalose, borate, and vitamin E to form a stable formula with enhanced safety, ocular adhesion, and hydration [125]. Other studies are briefly described in Table III. Figure 6 shows different nanostructured platforms [8].
Illustration of numerous nanostructured platform [8]
Niosomes
Niosomes are bilayered nanocarriers composed of self-aggregated non-ionic surfactants. They are biodegradable, biocompatible, enclose both hydrophilic and lipophilic drugs and non-immunogenic. They could prolong drug release and enhance its permeability and efficacy [161, 162]. Disadvantages of niosomes include chemical instability and possible hydrolysis, accumulation or loss of drug [18]. Cholesterol or its derivative is added to improve rigidity and stability of niosomes [8]. Elmotasem and Awad developed a niosomal system composed of span 60, cholesterol, poloxamer 407, hydroxypropyl methylcellulose, cyclodextrin, and chitosan. The resulted formula showed high drug entrapment, enhanced corneal permeation and activity [48]. Kaur et al. studied niosomal system composed of span 60, cholesterol, and chitosan. The finished product revealed higher activity, reduced side effects, and prolonged release [129]. Aggarwal et al. improved the duration of action and efficacy of acetazolamide using span 60, cholesterol, and Carbopol® 934P [130]. Zubairu et al. developed a niosomal system of gatifloxacin composed of span 60, cholesterol, and chitosan. The optimized formula showed enhanced antimicrobial activity, no toxicity, and superior ocular permeation [49]. More investigations are concisely mentioned in Table III.
Nanoemulsions
They are potential carriers for ocular delivery. Oils in water nanoemulsions are composed of dispersed oil phase that is stabilized by surfactants in an aqueous medium. They provide a reservoir for lipophilic drugs and interact with the lipids of tear film providing a sustained drug release [87, 163]. Surfactants are important for the interaction with the surface of the cornea, plus enhancing drug solubility [8]. Drawbacks of nanoemulsions include blurred vision if the particle size exceeds 100 nm due to development of milky formulation and reduced ocular tolerance due to high surfactant concentration [163]. Akhter et al. developed nanoemulsions system of cyclosporine A. Many oils, chitosan, Carbopol®, and Transcutol® P were incorporated. The resulted formula revealed enhanced drug retention, safety, and efficacy [87]. Oleic acid, polysorbate 80, poloxamer 188, chitosan, and polymyxin B were used by Bazán Henostroza et al., to improve stability, mucoadhesion, and antibiotic activity of rifampicin [50]. Soltani et al. constructed nanoemulsions of ketotifen fumarate utilizing Eudragit® RL 100 and polyvinyl alcohol. Enhanced corneal permeation and sustained release were obtained [88]. Additional findings are listed in Table III.
Nanosuspensions
They are colloidal nanocarriers constituted of lipophilic or semi-lipophilic drugs, suspended in a dispersion medium and stabilized by surfactants or polymers [121]. Their advantages include sustained drug release, increased residence time, and enhanced drug solubility and bioavailability [161]. The most commonly used mucoadhesive agents in nanosuspensions are Eudragit® polymers. Pignatello et al. developed a nanosuspensions of cloricromene composed of Eudragit® RS and RL 100 and Tween 80. The resulted formula showed enhanced stability, corneal residence time, and permeation [136]. Ahuja et al. used Eudragit® S100 and poloxamer 188 to form nanosuspensions of diclofenac with enhanced percent entrapment, prolonged release, and increased anti-inflammatory activity [83]. Khan et al. employed Eudragit® RL100 to increase percent entrapment, sustain drug release, and enhance pilocarpine activity [137]. Extra results are briefly listed in Table III.
Nanomicelles
They are nanocarriers composed of anionic, cationic, or zwitterionic surfactants. They may be spherical, cylindrical, or star-shaped. They could entrap both hydrophilic and lipophilic drugs. They have simple preparation techniques, reduced toxicity, increased bioavailability, increased stability, and enhanced permeation. They could deliver drugs to both segments of the eye (anterior and posterior portions) [161]. Yingfang et al. developed nanomicelles of pimecrolimus using polyethylene glycol and poly (ε-caprolactone) as co-polymers. The resulted formula showed enhanced percent entrapment, sustained release, and enhanced activity [141]. Liu et al. enhanced ocular permeation and prolonged release of tacrolimus utilizing amino-terminated poly(ethylene glycol)-block-poly(D,L)-lactic acid and hydroxypropyl methylcellulose [142]. Terreni et al. used hyaluronic acid to sustain the release, increase permeation, and activity of cyclosporine A [143]. Table III briefly lists additional studies.
Polymeric Nanoparticles
Polymeric nanoparticles could be divided according to their structure and preparation method into nanospheres and nanocapsules. Nanospheres are small solid spheres composed of a dense polymeric network. They have a matrix type composition with a great surface area. The drug could be adsorbed on the surface or entrapped within the particle. However, nanocapsules are a small liquid core enclosed by a polymeric membrane. The drug could be adsorbed on the capsule surface or entrapped within the liquid core [121]. Polymeric nanoparticles could reach both segments of the eye. They improve patient compliance particularly in chronic complaints due to their small particle size. They have a prolonged drug release, improved permeation, and reduced elimination rate [161]. Yu et al. developed polymeric nanoparticles for dexamethasone utilizing glycol chitosan, N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride, and N-hydroxysuccinimide. They showed enhanced retention time, sustained release, and small particle size [51]. Bodoki et al. sustained the release and enhanced the efficacy of lutein using poly(lactic-co-glycolic acid), tween 80, and Poloxamer 407 [164]. Abdel-Rashid et al. enhanced ocular permeation and efficacy of acetazolamide employing chitosan, span 60, Tween® 80/20, and sodium tripolyphosphate [145]. More examples are concisely mentioned in Table III.
Solid Lipid Nanoparticles
They are a solid lipid matrix enclosing hydrophilic and lipophilic drugs [161]. Examples of lipids used to prepare solid lipid nanoparticles include triglycerides, fatty acids, steroids, and waxes. They do not require organic solvents since surfactants stabilize the lipid dispersion [165]. They are biodegradable, biocompatible, safe, and of low-cost preparation [165]. They showed enhanced ocular retention time, permeability, prolonged release, and improved bioavailability [161]. Ahmad et al. developed solid lipid nanoparticles loaded with etoposide employing Gelucire® 44/14 and Compritol® ATO 888. The resulted formula demonstrated sustained release, improved safety, and activity [149]. Tatke et al. constructed triamcinolone acetonide-loaded solid lipid nanoparticles utilizing Pluronic® F-68 and gellan gum. The finished formula ensured improved residence time and increased delivered concentration [150]. A mucoadhesive solid lipid nanoparticles of tobramycin was successfully examined by Chetoni et al. The system composed of stearic acid, Epikuron 200, and sodium taurocholate. Higher concentration of tobramycin in both segments of the eye was demonstrated [151].
Nanostructured Lipid Carriers
They are considered a second generation of lipid nanoparticles, composed of around 30% of liquid lipids but the finished formula is solid, with no crystalline structure [161]. The liquid oil droplets provide additional space for drug in lipid matrix leading to higher drug content compared to solid lipid nanoparticles. They show controlled release, small toxicity, and enhanced activity. Aytekin et al. studied nanostructured lipid carriers loaded with riboflavin utilizing Compritol® ATO 888, Gelucire® 44/14, Miglyol® 812, Cremophor® EL, Transcutol® P, and stearylamine. The finished product demonstrated superior corneal residence time, permeation, and safety [153]. Pai and Vavia constructed etoposide-loaded nanostructured lipid carriers using many solid and liquid lipids, glyceryl stearyl citrate, and chitosan. The resulted formula reveled sustained and improved activity [154]. Yu et al. used Compritol® 888 ATO, Miglyol® 812 N, Cremophor® EL, soy lecithin, carboxymethyl chitosan, genipin, and poloxamer F127 to formulate nanostructured lipid carriers of baicalin. Investigations showed increased corneal permeation, retention time, and safety [166]. More investigations are succinctly stated in Table III.
Nanocrystals
The drug represents a major composition of nanocrystals, being enclosed and stabilized by other excipients. They have small particle size, simple formation techniques, high mucoadhesion properties, and improved bioavailability [162]. Tuomela et al. created brinzolamide-loaded nanocrystals using poloxamer F68/ F127, polysorbate 80, and hydroxypropyl methycellulose. The finished formula revealed immediate dissolution and improved efficacy [157]. Romero et al. developed cationic nanocrystals of dexamethasone and polymyxin B using benzalkonium chloride and cetylpyridinium chloride. The resulted preparation revealed small particle size, enhanced retention time, and safety [158]. Orasugh et al. formulated a cellulose nanocrystals of pilocarpine. Sustained drug release and safety were demonstrated [167]. Nanocrystals could be promising nanocarriers to be investigated in the near future in details.
Dendrimers
They are star-shaped or tree-shaped highly branched 3D structure, composed of repetitive molecules enclosing a central core [162]. They are suitable for delivery of both hydrophilic and lipophilic drugs due to their several terminal groups [161]. They showed increased residence time, prolonged activity, improved bioavailability, targeted delivery, and antimicrobial properties. They could transfer medications to both segments of the eye [8]. Lancina et al. developed brimonidine tartrate-loaded dendrimers using methoxy-polyethylene glycol. Sustained release and improved activity were achived [168]. Mishra and Jain studied dendrimers entrapping acetazolamide. Increased residence time, prolonged release, and activity were confirmed [169]. Holden et al. developed timolol maleate-loaded dendrimers utilizing polyethylene glycol. The finished formula showed improved permeation and increased cellular uptake [170]. Table III clarifies briefly more studies about dendrimers.
Cubosomes
They are bicontinuous cubic liquid crystalline nanocarriers constructed by emulsification of lipids in water with the aid of stabilizer. They are stable, entrap high amount of drugs due to its large surface area, easy to prepare, biodegradable, and relatively safe [17]. El deep et al. formulated brimonidine tartrate-loaded cubosomes utilizing glyceryl monooleate and poloxamer 407. The resulted formula revealed sustained release, improved permeation, and bioavailability [17]. Younes et al. developed sertaconazole nitrate-loaded cubosomes using DL-α-Monoolein, pluronic® F127, Brij® 58, pluronic® F108, Tween 80, and polyvinyl alcohol. Improved permeation, stability, and efficacy were achieved [33]. Gaballa et al. developed cubosomal system of beclomethasone dipropionate employing glyceryl monooleate. Improved corneal permeation and anti-inflammatory activity were demonstrated [91].
Olaminosomes
Olaminosomes are mainly formed of oleic acid, oleylamine, and surfactant. Oleic acid is natural unsaturated free fatty acid. Oleic acid is safe, biodegradable, and biocompatible. Thus, oleic acid is often used in the preparation of ocular nanocarriers [12]. Oleylamine is an unsaturated fatty amine derived from oleic acid. It has the extensively used as surfactant or co-stabilizer. It is generally used in food and drug products as a result of its well-accepted safety [171]. Olaminosomes have a small particle size, high drug entrapment ability, improved corneal permeation, safety, and activity. Abd-Elsalam and ElKasabgy developed agomelatine-loaded olaminosomes. The optimum formula showed enhanced permeation and improved activity [12].
Bilosomes
Bilosomes are bilayered nanocarriers containing bile salts. They have high drug entrapment, minute particle size, accepted zeta potential, accepted safety, enhanced corneal permeation, and activity. Abdelbary et al. developed terconazole-loaded bilosomes using cholesterol, span 60, and edge activator. The resulted formula showed great entrapment, improved permeation, and enhanced activity [40].
Characterization of Nanocarriers
Visual Appearance
Figure 7 shows briefly the approaches used to characterize ocular nanocarriers. Visual appearance depends on the particle size, surfactant, and oil concentration and type. Nanosystems could be transparent, translucent to milky white. Transparency is estimated by percentage transmittance (% T) using a UV spectrophotometer at 520 nm [6]. Small particles permit light transmission resulting in translucent or transparent appearance. High % T indicates absence of visual disturbance. However, gelation would reduce the transparency by 15% [172].
Stability
Stability of different nanosystems could be determined through short-term stability (3 months), centrifugation test, heating–cooling cycle, freeze–thaw cycles, and storage at elevated temperatures. All tests are followed by visual evaluation [6, 36]. The structure of the constructed formula determine the storage condition which could be at ambient temperature (25 ± 2°C) [33, 34, 36] or refrigerated (4–8°C) [31, 173].
Size and Uniformity Analysis
Particle size (PS) and poly-dispersity index (PDI) are the determined variable. They are estimated by dynamic light scattering (DLS) or photon correlation spectroscopy (PCS) using either Zetasizer devices (Malvern) or Coulter Counter particle size analyzer [6]. The ratio of the standard deviation to the mean droplet size is known as a PDI. Regarding PDI, a value of 0 indicates homogenous system, while a value of 1 indicates heterogeneous system [174]. Generally, small PS and PDI are desirable for ocular drug delivery since they increase patient compliance and enhance corneal permeability and corneal bioavailability [33]. PS is affected by homogenization time, surfactants type, surfactants amount, lipids type, and lipids quantity. Using high amount of lipid would increase the viscosity of the medium resulting in high difficulty to break the particles and hence large PS [33]. However, high surfactant concentration would allow more coverage for the surface of nanosystem; consequently prevent additional growth in the PS [12]. Using surfactant with low hydrophilic-lipophilic balance (HLB) value would increase the hydrophobicity of the medium and decrease the free energy resulting in smaller PS [41]. Increasing the homogenization time would reduce the PS [33]. However, the efficacy of sonication process might be reduced if the fatty acid had a high melting point as a result of the increased viscosity of the formula [175].
Zeta Potential
Zeta potential (ZP) is an indicator of physical stability of the formed nanosystem. It is determined through electrophoretic movement of particles in an electrical field. Generally, ZP around ± 20 mV is appropriate for electrostatic attachment with the cornea surface. In addition, ZP ensures the stability because of electrostatic repulsion between the particles. It is high recommended to dilute the formed nanosystem prior to ZP determination [6]. Effective precorneal retention time is achieved when the absolute value of ZP lies between 20 and 40 mV. It has been demonstrated that ZP value + 40 mV of Catioprost (Latanoprost — cationic emulsion) revealed a comparable effect as Xalatan (commercial eye solution) for reducing IOP but have a superior ocular tolerance profile [176]. Also, cubosomal formula with ZP = −30.2 mV showed better bioavailability and activity compared to Alphagan P® eye drops [17].
Morphological
Transmission electron microscopy (TEM) and atomic force microscopy (AFM) approaches are valuable to ensure the results of dynamic light scattering (DLS) or photon correlation spectroscopy (PCS) [173]. TEM of the nanoemulsion referred by Tayel et al. showed spherical and homogenous structure with no aggregates. TEM was in harmony with the results obtained by PCS [135]. In addition to, the AFM examination of the nanoemulsion referred by Dukovski et al. revealed spherical structure with the same size as resulted from PCS [177]. TEM of the mixed micelles constructed by Younes et al. revealed spherical shape, with no accumulation and was comparable to DLS [34].
Refractive Index
The refractive index (RI) is detected by Abbe’s refractometer and employed to detect the water content of soft contact lenses. It is important to confirm that the nanosystem will not cause a blurred vision [178]. The optimum RI for ocular delivery is <1.476 since the RI of tear fluid ranged from 1.34 to 1.36 [178]. Ismail et al. obtained acceptable RI (1.334 to 1.338) for the formed nanoemulsion [179]. The mixed micelles constructed by Fahmy et al. showed adequate RI (1.348) [36].
Surface Tension
Tate’s law indicates that there is a direct relation between drop volume and surface tension. The volume of the drop regulates the amount of drug that reaches the eye. Surface tension is measured by tensiometer. Generally, the most appropriate dose for ocular delivery is 5–15 μL. However, commercial eye drops give 25.1 and 56.4 μL. Surfactants could condense the droplet size [180]. A surface tension below 35 mN/m results in painful ocular administration, while high surface tension leads to minor film stability. For ocular delivery, surface tension between 40 and 50 mN/m is required [181]. Dukovski et al. discovered that both chitosan and ibuprofen could reduce surface tension as a result of their surface-active properties [177].
Rheological Measurement
Low viscosity nanosystem allows beneficial compliance with minor blinking pain. However, high viscosity nanosystem could prolong contact time, reduce frequency of dose, and increase bioavailability, but also results in patient discomfort [177]. The appropriate viscosity for ocular preparation is between 2 and 3 mPa.s [6].
Drug Distribution
Both percent entrapment efficiency (% EE) and percent drug loading (% DL) are used to examine diffusion of drug inside the nanosystems. % DL indicates the mass ratio of drugs to the mass of the nanosystem; however, % EE reflects the incorporation of drugs within the nanosystem during the formulation process. Generally, % DL depends on the structure and physical and chemical properties of the carrier material; however, % EE depends on drug hydrophobicity, molecular weight, and structure. Additionally, obtaining high %DL is more difficult than high % EE for most nanosystems [182]. Prior to determination of amount of the drug, the formula may be subjected to ultrafiltration, ultracentrifugation, gel filtration, or microdialysis [6, 34, 36]. Said et al. determined % EE of voriconazole-loaded cubosomes after ultracentrifugation [37]. % EE of rifampicin-loaded nanoemulsion and ibuprofen-loaded nanoemulsion was conducted after ultrafiltration [50, 177] Lin et al. estimated both % EE and % DL for the constructed micellar system [183].
pH Measurement
pH determination is important to ensure safety and efficacy of nanosystems. Acidic (pH < 4) or alkaline (pH > 10) solution would harm the eye [37]. Also, pH from 4 to 8 would significantly enhance drug permeation [31]. The pH of ocular preparation usually ranged from 3.50 to 8.50 [37]. pH of the formed cubosomes referred by Said et al. was (6.20 ± 0.01) [37]. Micellar system constructed by Fahmy et al. revealed acceptable pH value (7.41 ± 0.01) [36].
Isotonicity and Osmolality
Osmolality measurements are based on the colligative properties of tears or ocular nanosystem known as the freezing point, boiling point, vapor pressure, and osmotic pressure. Osmolality of open eyes is ranged from 231 to 446 mOsm/kg due to fluid evaporation. Ocular preparation with osmolality lower than 100 mOsm/kg or greater than 640 mOsm/kg was considered an eye irritant. Osmolality is restored within 1 or 2 min subsequent to administration of the non-isotonic preparation [6].
Ocular Retention
Ocular retention is important since it will reduce the frequency of doses and improve drug bioavailability. Ocular retention largely depends on surface area of nanosystem, since large surface area will enhance residence time. Ocular retention is determined by texture analysis method, modified balance method, fluorescence retention method, γ-scintigraphy, and rheological synergism after mixing with mucoadhesive polymer [6]. As a general rule, the force needed to detach eyelid during normal blink is about 0.2 N and 0.8 N during strong blink [184]. For chitosan-coated cyclosporine nanoemulsions, the resulted force of detachment was 0.153 N [87].
Ocular Biocompatibility
Draize Test
It’s a traditional in vivo test to detect possible irritation potential of the formed nanosystems. It may be also used for cosmetics [185]. Draize test relays on scoring system from 0 (no irritation) to 3 (inflammation and redness) for the cornea, iris, and conjunctivae [18]. For example, Ismail et al. utilized the test on rabbits to compare between nanoemulsions of travoprost and Travatan® eye drops. Safety of the constructed formula was confirmed [179]. Also Eldeep et al. used Draize test to confirm safety of the topically applied niosomes of brimonidine tartrate against Alphgan P® [18].
Hen’s Egg Test
Because of the existence similarities between chorioallantoic membrane (CAM) and vascularization of mucosal tissue of humans, this technique is used to detect possible ocular irritation from nanosystem. The score is given based on clotting, bleeding, and hyperemia on CAM blood vessels [186]. For example, Mahboobian et al. examined the safety of the formed nanoemulsion versus negative control (PBS, pH = 7.4) and positive control (sodium dodecyl sulfate). Study was accomplished on freshly fertilized hens egg at 37 ± 0.5°C and relative humidity of 67 ± 5% RH for 10 days with regular rotation every 12 h. Irritation consequences such as hemorrhage or hypermia were evaluated by visual inspection. Safety of the nanoemulsion was demonstrated by the end of the experiment [186].
Corneal Permeation
Ability of the nanosystem to penetrate through cornea is studied through various in vivo, ex vivo, and in vitro tests. The in vivo models usually utilized the rodents (rabbit, rat, or mouse); however, in vitro and ex vivo models used epithelial cells layer cultures, reconstructed cornea, or excised cornea [6]. Also, different permeation chambers are available like Franz-type diffusion cell, modified Franz diffusion cell, modified using chamber, horizontal perfusion cells, modified Erlenmeyer flask diffusion cell, and polycarbonate corneal perfusion chamber [6]. Different permeation parameters are estimated to evaluate the permeation potential of nanosystem. Permeation parameters include the amount of drug permeated per unit area (µg/cm2), average flux (Jmax), permeability coefficient, and the enhancement ratio (ER) [5, 34].
Possible Interactions
Differential scanning calorimetry (DSC) and Fourier transform infrared spectroscopy (FTIR) are important techniques to detect possible interaction between the components of nanosystems [173]. They also assure complete entrapment of the drug. For example, the specific peaks of vancomycin, poly (d, l-lactide- coglycolide), and Eudragit® RS 100 were preserved ensuring the absence of chemical interactions [187], while complete entrapment of dorzolamide hydrochloride was confirmed by disappearance of its characteristic peak from DSC thermogram [16].
Approaches to Enhance Ocular Delivery
Improvement of Corneal Permeability
Figure 8 shows concisely the approaches used to enhance ocular delivery. One of the approaches to enhance drug bioavailability following topical administration is increasing corneal permeability. For example, changing membrane components and/or disrupting epithelial tight junctions using surfactants, permeation enhancers, calcium chelating agents [188], and modifying physicochemical characters of the ionized drug using ion pairs [189]. On the other hand, enzymatic transformation of prodrug would convert it into the active after appropriate permeation [190]. Finally, applying a low-intensity electrical current (iontophoresis) would enhance drug permeation by electrorepulsion and electroosmosis effects.
Improvement of Corneal Retention Time
One of the techniques to increase corneal retention time is inclusion of excipients. Excipients could be a viscosity increasing polymers. However, high viscous eye drops are irritating for many patients, do not provide an accurate dose and result in blurred vision [191]. In situ gel has sustained contact time compared to simple solutions [117]. There are three types of in situ gel according to the transition properties: temperature, ionic, or pH sensitive [118]. In situ gel of ciprofloxacin with hydroxypropyl methylcellulose and sodium alginate (ion-sensitive) showed enhanced residence time and sustained drug release [98]. More examples of in situ gel were previously mentioned in Table II. The mucus gel layer covering the ocular surface is made of mucins, a class of at least 20 O-glycosyl proteins with anionic charge. Excipients permitting attachment to this mucus gel layer provide a sustained residence time [191]. Chitosan is a commonly used mucoadhesive polymer by virtue of its polycationic nature and the existence of many reactive amino groups capable of interactacting with mucin layer. Kaur et al. studied niosomal system composed of span 60, cholesterol, and chitosan which revealed higher activity, reduced side effects, and prolonged release [129]. Cyclodextrins are widely used cyclic glycopyranose oligosaccharides. They have the ability to enhance drug solubility and to attach covalently to mucoadhesive polymers to prolong residence time [191]. Sayed et al. used β-cyclodextrin to enhance ocular delivery of itraconazole [35]. Many colloidal delivery nanosystems have been employed to enhance ocular delivery. They have the ability to carry different drugs, increase bioavailability, reduce frequency and potential side effects, and improve patient’s compliance. Different ocular nanosystems were previously stated in Table III. Also, solid polymeric devices have been developed as authorized sustained release ocular dosage forms. However, solid strategies are frequently not accepted by patients because of discomfort and interference with vision. Sustained bimatoprost activity for many months was proved after incorporation of its insert [110]. Dexamethasone contact lens was prepared by encapsulation technique. It showed 200 fold drug retention in the retina matching with conventional eye drops [116]. More examples of solid dosage forms were formerly listed in Table II.
Future Technologies
Smart Nano-Micro Platforms
Smart denotes to nano-micro matrix that can considerably change their mechanical, thermal, and/or optical properties in a manageable or expectable means, and they can achieve sensing triggering roles with stimuli-responsive features. Unlike conventional nanocarriers, the smart nano-micro platforms can reveal precise reaction to exogenous (light, sound, and magnetic field) or endogenous (pH, reactive oxygen species, and biological molecules such as DNA and enzymes) factors resulting in accomplishing many functions, e.g., site-specific drug delivery, bio-imaging, and detection of bio-molecules. These fascinating techniques have extended into ocular delivery in recent years. Generally, these revolutionary systems have been used for cancer diagnosis and management, to enhance the bioavailability of drugs/agents, minimize side effects, and augment safety and efficacy [192, 193]. Tsujinaka et al. successfully delivered sunitinib microparticles that effectively inhibit the intraocular inflammation in mice model up to 6 months [194]. Rodriguez et al. constructed solid lipid nanoparticles that carry miRNA as gene therapy [195]. Basuk et al. demonstrated photo-modulated release of pre-loaded bevacizumab using visible light [196].
Extracellular Vesicles (Exosomes)
Extracellular vesicles are a sort of organelle that is produced by different cell types. Various bioactive compounds for example proteins, lipids, RNAs, and DNAs are enclosed within extracellular vesicles. They have a nano-size behave as a strong intercellular trigger that can start different physiological and pathological consequences. Under pathological situations, they could be produced by immune cells and control the inflammation progressions. They have a well-recognized role in immune-mediated eye diseases, such as Sjogren’s syndrome and corneal allograft rejection [197]. Also, they could encourage renewal of corneal tissue by stimulating the production of different matrix components. Additional investigations are required to develop ocular delivery systems based on exosomes. Tang et al. constructed exosomes of pluripotent stem cell-derived mesenchymal stem cells to hasten the restorative process of the corneal epithelium [198]. Zhu et al. developed exosomes derived from lens epithelial cells to load doxorubicin to prevent posterior capsular opacification [199].
Tissue Engineering
Tissue engineering investigations are classified into two types. First type is additive tissue engineering which substitutes cells or tissue or tries to permit the growth of something that is no longer there. The second type is arrestive tissue engineering that prevents irregular growth. Both additive and arrestive tissue engineering could be performed utilizing nanosystems. Examples of nanosystem-based tissue engineering include check of retinal ganglion cell viability [200], retinal ganglion cell repair [201], formulation of nanofiber scaffolds [202], corneal endothelial cell transplantation [203], and inhibition of retinal cell apoptosis [204]. Scientists begun to examine if nanotools and nanomaterials could be used to restore neural function of eye’s nerve cells.
Innovations in Clinical Trials
Continuous clinical trials for different dosage forms give the lead for pioneer treatment. For example, pilocarpine topical cream (semi-solid) for the treatment of presbyopia. It is a multicenter, randomized, double-masked, placebo-controlled, parallel group phase 2 trial evaluating the safety and efficacy of the cream. The study starts at January 3, 2022 and will continue till May 2023. Moreover, Cequa™ (Cyclosporine) ophthalmic emulsion (twice daily). This is a phase 4, multicenter, single arm, and 12-week study. An example of solid dosage form includes Dextenza 0.4 Mg (dexamethasone) ophthalmic insert. The study is performed to assess the efficacy and safety of Dextenza insert for the treatment of pain and inflammation following corneal transplant surgery.
Conclusions
The effective management of ophthalmic diseases remains a difficult mission as a result of existence of many ocular obstacles in the anterior and posterior sections of the eye. There are many ocular routes of administration that are used in order to deliver the medication into the targeted site of action such as topical, intraocular, periocular, or in conjugation with ocular devices. Several approaches and technologies have been adopted in order to minimize dosing interval, administrated dose, and unwanted effects and to enhance ocular retention time, drug permeation efficacy, and ocular bioavailability via controlled and sustained drug delivery systems. These advanced technologies have improved drug efficacy and shown good biocompatibility which suggest that they might have wide applications in the management and treatment of ocular diseases. In the future, more innovations are predicated in the ocular drug delivery systems in order to enhance and preserve the health of the eye, to improve patient compliance, and to accomplish superior results in the management of ocular diseases.
Data Availability
Data is available within the article.
References
Krishnaswami V, Kandasamy R, Alagarsamy S, Palanisamy R, Natesan S. Biological macromolecules for ophthalmic drug delivery to treat ocular diseases. Int J Biol Macromol. 2018;110:7–16. https://doi.org/10.1016/j.ijbiomac.2018.01.120.
Chitra PS, Chaki D, Boiroju NK, Mokalla TR, Gadde AK, Agraharam SG, et al. Status of oxidative stress markers, advanced glycation index, and polyol pathway in age-related cataract subjects with and without diabetes. Exp Eye Res. 2020;200:108230. https://doi.org/10.1016/j.exer.2020.108230.
Leske MC. Open-angle glaucoma - an epidemiologic overview. Ophthalmic Epidemiol. 2007;14(4):166–72. https://doi.org/10.1080/09286580701501931.
Elsayed I, Sayed S. Tailored nanostructured platforms for boosting transcorneal permeation: Box-Behnken statistical optimization, comprehensive in vitro, ex vivo and in vivo characterization. Int J Nanomed. 2017;12:7947–62. https://doi.org/10.2147/IJN.S150366.
Ahmed S, Amin MM, El-Korany SM, Sayed S. Corneal targeted fenticonazole nitrate-loaded novasomes for the management of ocular candidiasis: preparation, in vitro characterization, ex vivo and in vivo assessments. Drug Deliv. 2022;29(1):2428–41. https://doi.org/10.1080/10717544.2022.2103600.
Singh M, Bharadwaj S, Lee KE, Kang SG. Therapeutic nanoemulsions in ophthalmic drug administration: concept in formulations and characterization techniques for ocular drug delivery. J Control Release. 2020;328:895-916. https://doi.org/10.1016/j.jconrel.2020.10.025.
Maulvi FA, Shetty KH, Desai DT, Shah DO, Willcox MDP. Recent advances in ophthalmic preparations: ocular barriers, dosage forms and routes of administration. Int J Pharm. 2021;608:121105. https://doi.org/10.1016/j.ijpharm.2021.121105.
Silva B, Sao Braz B, Delgado E, Goncalves L. Colloidal nanosystems with mucoadhesive properties designed for ocular topical delivery. Int J Pharm. 2021;606:120873. https://doi.org/10.1016/j.ijpharm.2021.120873.
Kels BD, Grzybowski A, Grant-Kels JM. Human ocular anatomy. Clin Dermatol. 2015;33(2):140–6. https://doi.org/10.1016/j.clindermatol.2014.10.006.
Randazzo J, Zhang P, Makita J, Blessing K, Kador PF. Orally active multi-functional antioxidants delay cataract formation in streptozotocin (type 1) diabetic and gamma-irradiated rats. PLoS ONE. 2011;6(4):e18980. https://doi.org/10.1371/journal.pone.0018980.
Lu A, Duan P, Xie J, Gao H, Chen M, Gong Y, et al. Recent progress and research trend of anti-cataract pharmacology therapy: a bibliometric analysis and literature review. Eur J Pharmacol. 2022;934:175299. https://doi.org/10.1016/j.ejphar.2022.175299.
Abd-Elsalam WH, ElKasabgy NA. Mucoadhesive olaminosomes: a novel prolonged release nanocarrier of agomelatine for the treatment of ocular hypertension. Int J Pharm. 2019;560:235–45. https://doi.org/10.1016/j.ijpharm.2019.01.070.
Stuart KV, Madjedi K, Luben RN, Chua SYL, Warwick AN, Chia M, et al. Alcohol, intraocular pressure and open-angle glaucoma: a systematic review and meta-analysis. Ophthalmology. 2022. https://doi.org/10.1016/j.ophtha.2022.01.023.
Allison K, Patel D, Alabi O. Epidemiology of glaucoma: the past, present, and predictions for the future. Cureus. 2020;12(11):e11686. https://doi.org/10.7759/cureus.11686.
Aslan M, Cort A, Yucel I. Oxidative and nitrative stress markers in glaucoma. Free Radic Biol Med. 2008;45(4):367–76. https://doi.org/10.1016/j.freeradbiomed.2008.04.026.
Sayed S, Abdelmoteleb M, Amin MM, Khowessah OM. Effect of formulation variables and gamma sterilization on transcorneal permeation and stability of proniosomal gels as ocular platforms for antiglaucomal drug. AAPS PharmSciTech. 2020;21(3):87. https://doi.org/10.1208/s12249-020-1626-2.
Emad Eldeeb A, Salah S, Ghorab M. Formulation and evaluation of cubosomes drug delivery system for treatment of glaucoma: ex-vivo permeation and in-vivo pharmacodynamic study. J Drug Deliv Sci Technol. 2019;52:236–47. https://doi.org/10.1016/j.jddst.2019.04.036.
Emad Eldeeb A, Salah S, Ghorab M. Proniosomal gel-derived niosomes: an approach to sustain and improve the ocular delivery of brimonidine tartrate; formulation, in-vitro characterization, and in-vivo pharmacodynamic study. Drug Deliv. 2019;26(1):509–21. https://doi.org/10.1080/10717544.2019.1609622.
Anderson OA, Bainbridge JW, Shima DT. Delivery of anti-angiogenic molecular therapies for retinal disease. Drug Discov Today. 2010;15(7–8):272–82. https://doi.org/10.1016/j.drudis.2010.02.004.
Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–16. https://doi.org/10.1016/S2214-109X(13)70145-1.
Birch DG, Liang FQ. Age-related macular degeneration: a target for nanotechnology derived medicines. Int J Nanomed. 2007;2(1):65–77. https://doi.org/10.2147/nano.2007.2.1.65.
Kourlas H, Schiller DS. Pegaptanib sodium for the treatment of neovascular age-related macular degeneration: a review. Clin Ther. 2006;28(1):36–44. https://doi.org/10.1016/j.clinthera.2006.01.009.
Agban Y, Thakur SS, Mugisho OO, Rupenthal ID. Depot formulations to sustain periocular drug delivery to the posterior eye segment. Drug Discov Today. 2019;24(8):1458–69. https://doi.org/10.1016/j.drudis.2019.03.023.
Glendenning A, Crews K, Sturdivant J, Kopczynski C, Lin C-W, de Long M. Sustained release, biodegradable PEA implants for intravitreal delivery of ROCK/PKC inhibitor AR-13503. 2018.
Hosoya K, Lee VH, Kim KJ. Roles of the conjunctiva in ocular drug delivery: a review of conjunctival transport mechanisms and their regulation. Eur J Pharm Biopharm. 2005;60(2):227–40. https://doi.org/10.1016/j.ejpb.2004.12.007.
Azari AA, Barney NP. Conjunctivitis: a systematic review of diagnosis and treatment. JAMA. 2013;310(16):1721–9. https://doi.org/10.1001/jama.2013.280318.
Coney JM, Scott AW. Racial disparities in the screening and treatment of diabetic retinopathy. J Natl Med Assoc. 2022. https://doi.org/10.1016/j.jnma.2021.12.011.
Bolinger MT, Antonetti DA. Moving past anti-VEGF: novel therapies for treating diabetic retinopathy. Int J Mol Sci. 2016;17(9). https://doi.org/10.3390/ijms17091498.
ElZomor H, Taha H, Aleieldin A, Nour R, Zaghloul MS, Fawzi M, et al. High risk retinoblastoma: prevalence and success of treatment in developing countries. Ophthalmic Genet. 2015;36(3):287–9. https://doi.org/10.3109/13816810.2015.1016241.
Corson TW, Samuels BC, Wenzel AA, Geary AJ, Riley AA, McCarthy BP, et al. Multimodality imaging methods for assessing retinoblastoma orthotopic xenograft growth and development. PLoS ONE. 2014;9(6):e99036. https://doi.org/10.1371/journal.pone.0099036.
Ahmed S, Amin MM, El-Korany SM, Sayed S. Pronounced capping effect of olaminosomes as nanostructured platforms in ocular candidiasis management. Drug Deliv. 2022;29(1):2945–58. https://doi.org/10.1080/10717544.2022.2120926.
Boomiraj H, Mohankumar V, Lalitha P, Devarajan B. Human corneal microRNA expression profile in fungal keratitis. Invest Ophthalmol Vis Sci. 2015;56(13):7939–46. https://doi.org/10.1167/iovs.15-17619.
Younes NF, Abdel-Halim SA, Elassasy AI. Corneal targeted Sertaconazole nitrate loaded cubosomes: preparation, statistical optimization, in vitro characterization, ex vivo permeation and in vivo studies. Int J Pharm. 2018;553(1–2):386–97. https://doi.org/10.1016/j.ijpharm.2018.10.057.
Younes NF, Abdel-Halim SA, Elassasy AI. Solutol HS15 based binary mixed micelles with penetration enhancers for augmented corneal delivery of sertaconazole nitrate: optimization, in vitro, ex vivo and in vivo characterization. Drug Deliv. 2018;25(1):1706–17. https://doi.org/10.1080/10717544.2018.1497107.
Sayed S, Elsayed I, Ismail MM. Optimization of beta-cyclodextrin consolidated micellar dispersion for promoting the transcorneal permeation of a practically insoluble drug. Int J Pharm. 2018;549(1–2):249–60. https://doi.org/10.1016/j.ijpharm.2018.08.001.
Fahmy AM, Hassan M, El-Setouhy DA, Tayel SA, Al-Mahallawi AM. Voriconazole ternary micellar systems for the treatment of ocular mycosis: statistical optimization and in vivo evaluation. J Pharm Sci. 2021;110(5):2130–8. https://doi.org/10.1016/j.xphs.2020.12.013.
Said M, Aboelwafa AA, Elshafeey AH, Elsayed I. Central composite optimization of ocular mucoadhesive cubosomes for enhanced bioavailability and controlled delivery of voriconazole. J Drug Deliv Sci Technol. 2021;61:102075. https://doi.org/10.1016/j.jddst.2020.102075.
Lee VH, Robinson JR. Mechanistic and quantitative evaluation of precorneal pilocarpine disposition in albino rabbits. J Pharm Sci. 1979;68(6):673–84. https://doi.org/10.1002/jps.2600680606.
Duvvuri S, Majumdar S, Mitra AK. Drug delivery to the retina: challenges and opportunities. Expert Opin Biol Ther. 2003;3(1):45–56. https://doi.org/10.1517/14712598.3.1.45.
Abdelbary AA, Abd-Elsalam WH, Al-Mahallawi AM. Fabrication of novel ultradeformable bilosomes for enhanced ocular delivery of terconazole: in vitro characterization, ex vivo permeation and in vivo safety assessment. Int J Pharm. 2016;513(1–2):688–96. https://doi.org/10.1016/j.ijpharm.2016.10.006.
Abdelbary GA, Amin MM, Zakaria MY. Ocular ketoconazole-loaded proniosomal gels: formulation, ex vivo corneal permeation and in vivo studies. Drug Deliv. 2017;24(1):309–19. https://doi.org/10.1080/10717544.2016.1247928.
Sayed S, Abdel-Moteleb M, Amin MM, Khowessah OM. Cubogel as potential platform for glaucoma management. Drug Deliv. 2021;28(1):293–305. https://doi.org/10.1080/10717544.2021.1872740.
Liu S, Dozois MD, Chang CN, Ahmad A, Ng DL, Hileeto D, et al. Prolonged ocular retention of mucoadhesive nanoparticle eye drop formulation enables treatment of eye diseases using significantly reduced dosage. Mol Pharm. 2016;13(9):2897–905. https://doi.org/10.1021/acs.molpharmaceut.6b00445.
Zhang F, Chen H, Lan J, Song K, Wu X. Preparation and in vitro/in vivo evaluations of novel ocular micelle formulations of hesperetin with glycyrrhizin as a nanocarrier. Exp Eye Res. 2021;202:108313. https://doi.org/10.1016/j.exer.2020.108313.
Matsuda T, Hiraoka S, Urashima H, Ogura A, Ishida T. Preparation of an ultrafine rebamipide ophthalmic suspension with high transparency. Biol Pharm Bull. 2017;40(5):665–74. https://doi.org/10.1248/bpb.b16-00962.
Simta J, Kavita I, Milind B. Novel long retentive posaconazole ophthalmic suspension. Pharma Sci & Tech. 2020;4(1):1–10. https://doi.org/10.11648/j.pst.20200401.11.
Zhang J, Wang S. Topical use of coenzyme Q10-loaded liposomes coated with trimethyl chitosan: tolerance, precorneal retention and anti-cataract effect. Int J Pharm. 2009;372(1–2):66–75. https://doi.org/10.1016/j.ijpharm.2009.01.001.
Elmotasem H, Awad GEA. A stepwise optimization strategy to formulate in situ gelling formulations comprising fluconazole-hydroxypropyl-beta-cyclodextrin complex loaded niosomal vesicles and Eudragit nanoparticles for enhanced antifungal activity and prolonged ocular delivery. Asian J Pharm Sci. 2020;15(5):617–36. https://doi.org/10.1016/j.ajps.2019.09.003.
Zubairu Y, Negi LM, Iqbal Z, Talegaonkar S. Design and development of novel bioadhesive niosomal formulation for the transcorneal delivery of anti-infective agent: in-vitro and ex-vivo investigations. Asian J Pharm Sci. 2015;10(4):322–30. https://doi.org/10.1016/j.ajps.2015.02.001.
Bazán Henostroza MA, Curo Melo KJ, Nishitani Yukuyama M, Löbenberg R, Araci Bou-Chacra N. Cationic rifampicin nanoemulsion for the treatment of ocular tuberculosis. Colloids Surf A Physicochem Eng Asp. 2020;597:124755. https://doi.org/10.1016/j.colsurfa.2020.124755.
Yu A, Shi H, Liu H, Bao Z, Dai M, Lin D, et al. Mucoadhesive dexamethasone-glycol chitosan nanoparticles for ophthalmic drug delivery. Int J Pharm. 2020;575:118943. https://doi.org/10.1016/j.ijpharm.2019.118943.
Battiston K, Parrag I, Statham M, Louka D, Fischer H, Mackey G, et al. Polymer-free corticosteroid dimer implants for controlled and sustained drug delivery. Nat Commun. 2021;12(1):2875. https://doi.org/10.1038/s41467-021-23232-7.
Park JG, Callaway NF, Ludwig CA, Mahajan VB. Intravitreal methotrexate and fluocinolone acetonide implantation for Vogt-Koyanagi-Harada uveitis. Am J Ophthalmol Case Rep. 2020;19:100859. https://doi.org/10.1016/j.ajoc.2020.100859.
Abrishami M, Motamed Shariati M, Malaekeh-Nikouei B, Tajani AS, Mahmoudi A, Abrishami M, et al. Preparation and in vivo evaluation of nanoliposomes containing vancomycin after intravitreal injection in albino rabbits. Iran J Basic Med Sci. 2020;23(4):551–5. https://doi.org/10.22038/ijbms.2020.43447.10205.
Yiu G, Chung SH, Mollhoff IN, Nguyen UT, Thomasy SM, Yoo J, et al. Suprachoroidal and subretinal injections of AAV using transscleral microneedles for retinal gene delivery in nonhuman primates. Mol Ther Methods Clin Dev. 2020;16:179–91. https://doi.org/10.1016/j.omtm.2020.01.002.
Cocarta AI, Hobzova R, Sirc J, Cerna T, Hrabeta J, Svojgr K, et al. Hydrogel implants for transscleral drug delivery for retinoblastoma treatment. Mater Sci Eng C Mater Biol Appl. 2019;103:109799. https://doi.org/10.1016/j.msec.2019.109799.
Radhakrishnan K, Vincent A, Joseph RR, Moreno M, Dickescheid A, Agrawal R, et al. Hollow microcapsules as periocular drug depot for sustained release of anti-VEGF protein. Pharmaceutics. 2019;11(7). https://doi.org/10.3390/pharmaceutics11070330.
Safi M, Ang MJ, Patel P, Silkiss RZ. Rhino-orbital-cerebral mucormycosis (ROCM) and associated cerebritis treated with adjuvant retrobulbar amphotericin B. Am J Ophthalmol Case Rep. 2020;19:100771. https://doi.org/10.1016/j.ajoc.2020.100771.
Cosgrove R, Rossow T, Cosgrove M, Siegel M. Suspected systemic uptake of chlorpromazine after retrobulbar injection. Am J Ophthalmol Case Rep. 2020;19:100801. https://doi.org/10.1016/j.ajoc.2020.100801.
Hayashi K, Hayashi H. Intravitreal versus retrobulbar injections of triamcinolone for macular edema associated with branch retinal vein occlusion. Am J Ophthalmol. 2005;139(6):972–82. https://doi.org/10.1016/j.ajo.2004.12.087.
Wong CW, Czarny B, Metselaar JM, Ho C, Ng SR, Barathi AV, et al. Evaluation of subconjunctival liposomal steroids for the treatment of experimental uveitis. Sci Rep. 2018;8(1):6604. https://doi.org/10.1038/s41598-018-24545-2.
Salama HA, Ghorab M, Mahmoud AA, Abdel HM. PLGA Nanoparticles as subconjunctival injection for management of glaucoma. AAPS PharmSciTech. 2017;18(7):2517–28. https://doi.org/10.1208/s12249-017-0710-8.
Martinez-Carrasco R, Sanchez-Abarca LI, Nieto-Gomez C, Martin Garcia E, Sanchez-Guijo F, Argueso P, et al. Subconjunctival injection of mesenchymal stromal cells protects the cornea in an experimental model of GVHD. Ocul Surf. 2019;17(2):285–94. https://doi.org/10.1016/j.jtos.2019.01.001.
Cuming RS, Abarca EM, Duran S, Wooldridge AA, Stewart AJ, Ravis W, et al. Development of a sustained-release voriconazole-containing thermogel for subconjunctival injection in horses. Invest Ophthalmol Vis Sci. 2017;58(5):2746–54. https://doi.org/10.1167/iovs.16-20899.
Fu J, Sun F, Liu W, Liu Y, Gedam M, Hu Q, et al. Subconjunctival delivery of dorzolamide-loaded poly(ether-anhydride) microparticles produces sustained lowering of intraocular pressure in rabbits. Mol Pharm. 2016;13(9):2987–95. https://doi.org/10.1021/acs.molpharmaceut.6b00343.
Walter K, Delwadia N, Coben J. Continuous intracameral phenylephrine-ketorolac irrigation for miosis prevention in femtosecond laser-assisted cataract surgery: reduction in surgical time and iris manipulation. J Cataract Refract Surg. 2019;45(4):465–9. https://doi.org/10.1016/j.jcrs.2018.11.004.
Fan F, Zhao Z, Zhao X, Ma Q, Li K, Fu W, et al. Reduction of ocular surface damage and bacterial survival using 0.05% povidone-iodine ocular surface irrigation before cataract surgery. Ophthalmic Res. 2019;62(3):166–72. https://doi.org/10.1159/000501373.
Igarashi T, Ohsawa I, Kobayashi M, Igarashi T, Suzuki H, Iketani M, et al. Hydrogen prevents corneal endothelial damage in phacoemulsification cataract surgery. Sci Rep. 2016;6:31190. https://doi.org/10.1038/srep31190.
Chen Y, Kalia YN. Short-duration ocular iontophoresis of ionizable aciclovir prodrugs: a new approach to treat herpes simplex infections in the anterior and posterior segments of the eye. Int J Pharm. 2018;536(1):292–300. https://doi.org/10.1016/j.ijpharm.2017.11.069.
Cohen AE, Assang C, Patane MA, From S, Korenfeld M, Avion Study I. Evaluation of dexamethasone phosphate delivered by ocular iontophoresis for treating noninfectious anterior uveitis. Ophthalmology. 2012;119(1):66–73. https://doi.org/10.1016/j.ophtha.2011.07.006.
Dos Santos GA, Ferreira-Nunes R, Dalmolin LF, Dos Santos Re AC, Anjos JLV, Mendanha SA, et al. Besifloxacin liposomes with positively charged additives for an improved topical ocular delivery. Sci Rep. 2020;10(1):19285. https://doi.org/10.1038/s41598-020-76381-y.
See GL, Sagesaka A, Sugasawa S, Todo H, Sugibayashi K. Eyelid skin as a potential site for drug delivery to conjunctiva and ocular tissues. Int J Pharm. 2017;533(1):198–205. https://doi.org/10.1016/j.ijpharm.2017.09.070.
Chan KC, Yu Y, Ng SH, Mak HK, Yip YWY, van der Merwe Y, et al. Intracameral injection of a chemically cross-linked hydrogel to study chronic neurodegeneration in glaucoma. Acta Biomater. 2019;94:219–31. https://doi.org/10.1016/j.actbio.2019.06.005.
Sobaci G, Tuncer K, Tas A, Ozyurt M, Bayer A, Kutlu U. The effect of intraoperative antibiotics in irrigating solutions on aqueous humor contamination and endophthalmitis after phacoemulsification surgery. Eur J Ophthalmol. 2003;13(9–10):773–8. https://doi.org/10.1177/1120672103013009-1007.
Jung JH, Chiang B, Grossniklaus HE, Prausnitz MR. Ocular drug delivery targeted by iontophoresis in the suprachoroidal space using a microneedle. J Control Release. 2018;277:14–22. https://doi.org/10.1016/j.jconrel.2018.03.001.
Christopher K, Chauhan A. Contact lens based drug delivery to the posterior segment via iontophoresis in cadaver rabbit eyes. Pharm Res. 2019;36(6):87. https://doi.org/10.1007/s11095-019-2625-4.
Maulvi FA, Soni TG, Shah DO. A review on therapeutic contact lenses for ocular drug delivery. Drug Deliv. 2016;23(8):3017–26. https://doi.org/10.3109/10717544.2016.1138342.
Naguib SS, Hathout RM, Mansour S. Optimizing novel penetration enhancing hybridized vesicles for augmenting the in-vivo effect of an anti-glaucoma drug. Drug Deliv. 2017;24(1):99–108. https://doi.org/10.1080/10717544.2016.1233588.
Siegl C, Konig-Schuster M, Nakowitsch S, Koller C, Graf P, Unger-Manhart N, et al. Pharmacokinetics of topically applied tacrolimus dissolved in Marinosolv, a novel aqueous eye drop formulation. Eur J Pharm Biopharm. 2019;134:88–95. https://doi.org/10.1016/j.ejpb.2018.11.015.
Lorenzo-Veiga B, Sigurdsson HH, Loftsson T. Nepafenac-loaded cyclodextrin/polymer nanoaggregates: a new approach to eye drop formulation. Materials (Basel). 2019;12(2). https://doi.org/10.3390/ma12020229.
Güven UM, Yenilmez E. Olopatadine hydrochloride loaded Kollidon ® SR nanoparticles for ocular delivery: nanosuspension formulation and in vitro–in vivo evaluation. J Drug Deliv Sci Technol. 2019;51:506–12. https://doi.org/10.1016/j.jddst.2019.03.016.
Das S, Suresh PK. Nanosuspension: a new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to amphotericin B. Nanomedicine : Nanotechnol Biol Med. 2011;7(2):242–7. https://doi.org/10.1016/j.nano.2010.07.003.
Ahuja M, Dhake AS, Sharma SK, Majumdar DK. Diclofenac-loaded Eudragit S100 nanosuspension for ophthalmic delivery. J Microencapsul. 2011;28(1):37–45. https://doi.org/10.3109/02652048.2010.523794.
Li X, Muller RH, Keck CM, Bou-Chacra NA. Mucoadhesive dexamethasone acetate-polymyxin B sulfate cationic ocular nanoemulsion–novel combinatorial formulation concept. Pharmazie. 2016;71(6):327–33.
Raval N, Khunt D, Misra M. Microemulsion-based delivery of triamcinolone acetonide to posterior segment of eye using chitosan and butter oil as permeation enhancer: an in vitro and in vivo investigation. J Microencapsul. 2018;35(1):62–77. https://doi.org/10.1080/02652048.2018.1425750.
Mukhopadhyay S. Preparation and evaluation of erythromycin microemulsion for opthalmic drug delivery. J Adv Sci Res. 2020;11(1):22–6.
Akhter S, Anwar M, Siddiqui MA, Ahmad I, Ahmad J, Ahmad MZ, et al. Improving the topical ocular pharmacokinetics of an immunosuppressant agent with mucoadhesive nanoemulsions: formulation development, in-vitro and in-vivo studies. Colloids Surf B. 2016;148:19–29. https://doi.org/10.1016/j.colsurfb.2016.08.048.
Soltani S, Zakeri-Milani P, Barzegar-Jalali M, Jelvehgari M. Design of eudragit RL nanoparticles by nanoemulsion method as carriers for ophthalmic drug delivery of ketotifen fumarate. Iran J Basic Med Sci. 2016;19(5):550–60.
Aboali FA, Habib DA, Elbedaiwy HM, Farid RM. Curcumin-loaded proniosomal gel as a biofreindly alternative for treatment of ocular inflammation: in-vitro and in-vivo assessment. Int J Pharm. 2020;589:119835. https://doi.org/10.1016/j.ijpharm.2020.119835.
Wang X, Zhang Y, Huang J, Tian C, Xia M, Liu L, et al. A novel phytantriol-based lyotropic liquid crystalline gel for efficient ophthalmic delivery of pilocarpine nitrate. AAPS PharmSciTech. 2019;20(1):32. https://doi.org/10.1208/s12249-018-1248-0.
Gaballa SA, El Garhy OH, Moharram H, Abdelkader H. Preparation and evaluation of cubosomes/cubosomal gels for ocular delivery of beclomethasone dipropionate for management of uveitis. Pharm Res. 2020;37(10):198. https://doi.org/10.1007/s11095-020-02857-1.
Bao Q, Newman B, Wang Y, Choi S, Burgess DJ. In vitro and ex vivo correlation of drug release from ophthalmic ointments. J Control Release. 2018;276:93–101. https://doi.org/10.1016/j.jconrel.2018.03.003.
Mirzaeei S, Berenjian K, Khazaei R. Preparation of the potential ocular inserts by electrospinning method to achieve the prolong release profile of triamcinolone acetonide. Adv Pharm Bull. 2018;8(1):21–7. https://doi.org/10.15171/apb.2018.003.
Bertens CJF, Martino C, van Osch MC, Lataster A, Dias A, van den Biggelaar F, et al. Design of the ocular coil, a new device for non-invasive drug delivery. Eur J Pharm Biopharm. 2020;150:120–30. https://doi.org/10.1016/j.ejpb.2020.03.010.
Maulvi FA, Patil RJ, Desai AR, Shukla MR, Vaidya RJ, Ranch KM, et al. Effect of gold nanoparticles on timolol uptake and its release kinetics from contact lenses: in vitro and in vivo evaluation. Acta Biomater. 2019;86:350–62. https://doi.org/10.1016/j.actbio.2019.01.004.
Torres-Luna C, Hu N, Tammareddy T, Domszy R, Yang J, Wang NS, et al. Extended delivery of non-steroidal anti-inflammatory drugs through contact lenses loaded with vitamin E and cationic surfactants. Cont Lens Anterior Eye. 2019;42(5):546–52. https://doi.org/10.1016/j.clae.2019.04.011.
Bin Sahadan MY, Tong WY, Tan WN, Leong CR, Bin Misri MN, Chan M, et al. Phomopsidione nanoparticles coated contact lenses reduce microbial keratitis causing pathogens. Exp Eye Res. 2019;178:10–4. https://doi.org/10.1016/j.exer.2018.09.011.
Makwana SB, Patel VA, Parmar SJ. Development and characterization of in-situ gel for ophthalmic formulation containing ciprofloxacin hydrochloride. Results Pharma Sci. 2016;6:1–6. https://doi.org/10.1016/j.rinphs.2015.06.001.
Yang X, Trinh HM, Agrahari V, Sheng Y, Pal D, Mitra AK. Nanoparticle-based topical ophthalmic gel formulation for sustained release of hydrocortisone butyrate. AAPS PharmSciTech. 2016;17(2):294–306. https://doi.org/10.1208/s12249-015-0354-5.
Wadetwar RN, Agrawal AR, Kanojiya PS. In situ gel containing Bimatoprost solid lipid nanoparticles for ocular delivery: in-vitro and ex-vivo evaluation. J Drug Deliv Sci Technol. 2020;56. https://doi.org/10.1016/j.jddst.2020.101575.
Allam A, El-Mokhtar MA, Elsabahy M. Vancomycin-loaded niosomes integrated within pH-sensitive in-situ forming gel for treatment of ocular infections while minimizing drug irritation. J Pharm Pharmacol. 2019;71(8):1209–21. https://doi.org/10.1111/jphp.13106.
Schoenwald RD, Stewart P. Effect of particle size on ophthalmic bioavailability of dexamethasone suspensions in rabbits. J Pharm Sci. 1980;69(4):391–4. https://doi.org/10.1002/jps.2600690407.
Li Q, Li Z, Zeng W, Ge S, Lu H, Wu C, et al. Proniosome-derived niosomes for tacrolimus topical ocular delivery: in vitro cornea permeation, ocular irritation, and in vivo anti-allograft rejection. Eur J Pharm Sci. 2014;62:115–23. https://doi.org/10.1016/j.ejps.2014.05.020.
Pandey SS, Maulvi FA, Patel PS, Shukla MR, Shah KM, Gupta AR, et al. Cyclosporine laden tailored microemulsion-gel depot for effective treatment of psoriasis: in vitro and in vivo studies. Colloids Surf B Biointerfaces. 2020;186:110681. https://doi.org/10.1016/j.colsurfb.2019.110681.
Wagh VD, Inamdar B, Samanta M. Polymers used in ocular dosage form and drug delivery systems. Asian J Pharm. 2014;2(1).
Xu X, Al-Ghabeish M, Rahman Z, Krishnaiah YS, Yerlikaya F, Yang Y, et al. Formulation and process factors influencing product quality and in vitro performance of ophthalmic ointments. Int J Pharm. 2015;493(1–2):412–25. https://doi.org/10.1016/j.ijpharm.2015.07.066.
Nguyen ET, Shorstein NH. Preparation of intracameral antibiotics for injection. J Cataract Refract Surg. 2013;39(11):1778–9. https://doi.org/10.1016/j.jcrs.2013.08.036.
Heralgi MM, Badami A, Vokuda H, Venkatachalam K. An update on voriconazole in ophthalmology. Off Sci J Delhi Ophthalmol Soc. 2016;27(1):9–15.
Kumari A, Sharma PK, Garg VK, Garg G. Ocular inserts - advancement in therapy of eye diseases. J Adv Pharm Technol Res. 2010;1(3):291–6. https://doi.org/10.4103/0110-5558.72419.
Brandt JD, DuBiner HB, Benza R, Sall KN, Walker GA, Semba CP, et al. Long-term safety and efficacy of a sustained-release bimatoprost ocular ring. Ophthalmology. 2017;124(10):1565–6. https://doi.org/10.1016/j.ophtha.2017.04.022.
Desai AR, Maulvi FA, Desai DM, Shukla MR, Ranch KM, Vyas BA, et al. Multiple drug delivery from the drug-implants-laden silicone contact lens: addressing the issue of burst drug release. Mater Sci Eng C Mater Biol Appl. 2020;112:110885. https://doi.org/10.1016/j.msec.2020.110885.
Hui A, Willcox M. In vivo studies evaluating the use of contact lenses for drug delivery. Optom Vis Sci. 2016;93(4):367–76. https://doi.org/10.1097/OPX.0000000000000809.
Hsu KH, Carbia BE, Plummer C, Chauhan A. Dual drug delivery from vitamin E loaded contact lenses for glaucoma therapy. Eur J Pharm Biopharm. 2015;94:312–21. https://doi.org/10.1016/j.ejpb.2015.06.001.
Maulvi FA, Parmar RJ, Desai AR, Desai DM, Shukla MR, Ranch KM, et al. Tailored gatifloxacin Pluronic(R) F-68-loaded contact lens: addressing the issue of transmittance and swelling. Int J Pharm. 2020;581:119279. https://doi.org/10.1016/j.ijpharm.2020.119279.
Maulvi FA, Parmar RJ, Shukla MR, Desai AR, Desai DT, Ranch KM, et al. Plackett-Burman design for screening of critical variables and their effects on the optical transparency and swelling of gatifloxacin-Pluronic-loaded contact lens. Int J Pharm. 2019;566:513–9. https://doi.org/10.1016/j.ijpharm.2019.06.008.
Ross AE, Bengani LC, Tulsan R, Maidana DE, Salvador-Culla B, Kobashi H, et al. Topical sustained drug delivery to the retina with a drug-eluting contact lens. Biomaterials. 2019;217:119285. https://doi.org/10.1016/j.biomaterials.2019.119285.
Mohanty D, Bakshi V, Simharaju N, Haque MA, Sahoo CK. A review on in situ gel: a novel drug delivery system. Int J Pharm Sci Rev Res. 2018;50(1):175–81.
Ranch KM, Maulvi FA, Koli AR, Desai DT, Parikh RK, Shah DO. Tailored doxycycline hyclate loaded in situ gel for the treatment of periodontitis: optimization, in vitro characterization, and antimicrobial studies. AAPS PharmSciTech. 2021;22(3):77. https://doi.org/10.1208/s12249-021-01950-x.
Z MAF, Vangala A, Longman M, Khaled KA, Hussein AK, El-Garhy OH, et al. Poloxamer-based thermoresponsive ketorolac tromethamine in situ gel preparations: design, characterisation, toxicity and transcorneal permeation studies. European Journal of Pharmaceutics and Biopharmaceutics : Official Journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. Eur J Pharm Biopharm. 2017;114:119–34. https://doi.org/10.1016/j.ejpb.2017.01.008.
Abed N, Couvreur P. Nanocarriers for antibiotics: a promising solution to treat intracellular bacterial infections. Int J Antimicrob Agents. 2014;43(6):485–96. https://doi.org/10.1016/j.ijantimicag.2014.02.009.
Ali J, Fazil M, Qumbar M, Khan N, Ali A. Colloidal drug delivery system: amplify the ocular delivery. Drug Deliv. 2016;23(3):710–26. https://doi.org/10.3109/10717544.2014.923065.
Rawas-Qalaji M, Williams CA. Advances in ocular drug delivery. Curr Eye Res. 2012;37(5):345–56. https://doi.org/10.3109/02713683.2011.652286.
Lin J, Wu H, Wang Y, Lin J, Chen Q, Zhu X. Preparation and ocular pharmacokinetics of hyaluronan acid-modified mucoadhesive liposomes. Drug Deliv. 2016;23(4):1144–51. https://doi.org/10.3109/10717544.2014.991952.
Cheng T, Li J, Cheng Y, Zhang X, Qu Y. Triamcinolone acetonide-chitosan coated liposomes efficiently treated retinal edema as eye drops. Exp Eye Res. 2019;188:107805. https://doi.org/10.1016/j.exer.2019.107805.
Vicario-de-la-Torre M, Caballo-Gonzalez M, Vico E, Morales-Fernandez L, Arriola-Villalobos P, De Las Heras B, et al. Novel nano-liposome formulation for dry eyes with components similar to the preocular tear film. Polymers (Basel). 2018;10(4). https://doi.org/10.3390/polym10040425.
Moustafa MA, El-Refaie WM, Elnaggar YSR, Abdallah OY. Gel in core carbosomes as novel ophthalmic vehicles with enhanced corneal permeation and residence. Int J Pharm. 2018;546(1–2):166–75. https://doi.org/10.1016/j.ijpharm.2018.05.040.
Tan G, Yu S, Pan H, Li J, Liu D, Yuan K, et al. Bioadhesive chitosan-loaded liposomes: a more efficient and higher permeable ocular delivery platform for timolol maleate. Int J Biol Macromol. 2017;94(Pt A):355–63. https://doi.org/10.1016/j.ijbiomac.2016.10.035.
Dong Y, Dong P, Huang D, Mei L, Xia Y, Wang Z, et al. Fabrication and characterization of silk fibroin-coated liposomes for ocular drug delivery. Eur J Pharm Biopharm. 2015;91:82–90. https://doi.org/10.1016/j.ejpb.2015.01.018.
Kaur IP, Aggarwal D, Singh H, Kakkar S. Improved ocular absorption kinetics of timolol maleate loaded into a bioadhesive niosomal delivery system. Graefes Arch Clin Exp Ophthalmol. 2010;248(10):1467–72. https://doi.org/10.1007/s00417-010-1383-0.
Aggarwal D, Pal D, Mitra AK, Kaur IP. Study of the extent of ocular absorption of acetazolamide from a developed niosomal formulation, by microdialysis sampling of aqueous humor. Int J Pharm. 2007;338(1–2):21–6. https://doi.org/10.1016/j.ijpharm.2007.01.019.
Abdelbary A, Salem HF, Khallaf RA, Ali AM. Mucoadhesive niosomal in situ gel for ocular tissue targeting: in vitro and in vivo evaluation of lomefloxacin hydrochloride. Pharm Dev Technol. 2017;22(3):409–17. https://doi.org/10.1080/10837450.2016.1219916.
El-Nabarawi MA, Abd El Rehem RT, Teaima M, Abary M, El-Mofty HM, Khafagy MM, et al. Natamycin niosomes as a promising ocular nanosized delivery system with ketorolac tromethamine for dual effects for treatment of candida rabbit keratitis; in vitro/in vivo and histopathological studies. Drug Dev Ind Pharm. 2019;45(6):922–36. https://doi.org/10.1080/03639045.2019.1579827.
Zeng W, Li Q, Wan T, Liu C, Pan W, Wu Z, et al. Hyaluronic acid-coated niosomes facilitate tacrolimus ocular delivery: mucoadhesion, precorneal retention, aqueous humor pharmacokinetics, and transcorneal permeability. Colloids Surf B. 2016;141:28–35. https://doi.org/10.1016/j.colsurfb.2016.01.014.
Gallarate M, Chirio D, Bussano R, Peira E, Battaglia L, Baratta F, et al. Development of O/W nanoemulsions for ophthalmic administration of timolol. Int J Pharm. 2013;440(2):126–34. https://doi.org/10.1016/j.ijpharm.2012.10.015.
Tayel SA, El-Nabarawi MA, Tadros MI, Abd-Elsalam WH. Promising ion-sensitive in situ ocular nanoemulsion gels of terbinafine hydrochloride: design, in vitro characterization and in vivo estimation of the ocular irritation and drug pharmacokinetics in the aqueous humor of rabbits. Int J Pharm. 2013;443(1–2):293–305. https://doi.org/10.1016/j.ijpharm.2012.12.049.
Pignatello R, Ricupero N, Bucolo C, Maugeri F, Maltese A, Puglisi G. Preparation and characterization of eudragit retard nanosuspensions for the ocular delivery of cloricromene. AAPS PharmSciTech. 2006;7(1):E27. https://doi.org/10.1208/pt070127.
Khan MS, Vishakante GD, Bathool A. Development and characterization of pilocarpine loaded Eudragit nanosuspensions for ocular drug delivery. J Biomed Nanotechnol. 2013;9(1):124–31. https://doi.org/10.1166/jbn.2013.1475.
Ahuja M, Verma P, Bhatia M. Preparation and evaluation of chitosan–itraconazole co-precipitated nanosuspension for ocular delivery. J Exp Nanosci. 2015;10(3):209–21.
Adibkia K, Siahi Shadbad MR, Nokhodchi A, Javadzedeh A, Barzegar-Jalali M, Barar J, et al. Piroxicam nanoparticles for ocular delivery: physicochemical characterization and implementation in endotoxin-induced uveitis. J Drug Target. 2007;15(6):407–16. https://doi.org/10.1080/10611860701453125.
Adibkia K, Omidi Y, Siahi MR, Javadzadeh AR, Barzegar-Jalali M, Barar J, et al. Inhibition of endotoxin-induced uveitis by methylprednisolone acetate nanosuspension in rabbits. J Ocul Pharmacol Ther. 2007;23(5):421–32. https://doi.org/10.1089/jop.2007.0039.
Yingfang F, Zhuang B, Wang C, Xu X, Xu W, Lv Z. Pimecrolimus micelle exhibits excellent therapeutic effect for Keratoconjunctivitis Sicca. Colloids Surf, B. 2016;140:1–10. https://doi.org/10.1016/j.colsurfb.2015.11.059.
Liu D, Wu Q, Chen W, Lin H, Zhu Y, Liu Y, et al. A novel FK506 loaded nanomicelles consisting of amino-terminated poly(ethylene glycol)-block-poly(D, L)-lactic acid and hydroxypropyl methylcellulose for ocular drug delivery. Int J Pharm. 2019;562:1–10. https://doi.org/10.1016/j.ijpharm.2019.03.022.
Terreni E, Chetoni P, Tampucci S, Burgalassi S, Al-Kinani AA, Alany RG, et al. Assembling surfactants-mucoadhesive polymer nanomicelles (ASMP-Nano) for ocular delivery of cyclosporine-A. Pharmaceutics. 2020;12(3). https://doi.org/10.3390/pharmaceutics12030253.
Xu X, Sun L, Zhou L, Cheng Y, Cao F. Functional chitosan oligosaccharide nanomicelles for topical ocular drug delivery of dexamethasone. Carbohydr Polym. 2020;227:115356. https://doi.org/10.1016/j.carbpol.2019.115356.
Abdel-Rashid RS, Helal DA, Omar MM, El Sisi AM. Nanogel loaded with surfactant based nanovesicles for enhanced ocular delivery of acetazolamide. Int J Nanomed. 2019;14:2973–83. https://doi.org/10.2147/IJN.S201891.
Fabiano A, Piras AM, Guazzelli L, Storti B, Bizzarri R, Zambito Y. Impact of different mucoadhesive polymeric nanoparticles loaded in thermosensitive hydrogels on transcorneal administration of 5-fluorouracil. Pharmaceutics. 2019;11(12). https://doi.org/10.3390/pharmaceutics11120623.
Alvarez-Trabado J, Lopez-Garcia A, Martin-Pastor M, Diebold Y, Sanchez A. Sorbitan ester nanoparticles (SENS) as a novel topical ocular drug delivery system: design, optimization, and in vitro/ex vivo evaluation. Int J Pharm. 2018;546(1–2):20–30. https://doi.org/10.1016/j.ijpharm.2018.05.015.
Upadhayay P, Kumar M, Pathak K. Norfloxacin loaded pH triggered nanoparticulate in-situ gel for extraocular bacterial infections: optimization, ocular irritancy and corneal toxicity. Iran J Pharm Res. 2016;15(1):3–22.
Ahmad I, Pandit J, Sultana Y, Mishra AK, Hazari PP, Aqil M. Optimization by design of etoposide loaded solid lipid nanoparticles for ocular delivery: characterization, pharmacokinetic and deposition study. Mater Sci Eng C Mater Biol Appl. 2019;100:959–70. https://doi.org/10.1016/j.msec.2019.03.060.
Tatke A, Dudhipala N, Janga KY, Balguri SP, Avula B, Jablonski MM, et al. In situ gel of triamcinolone acetonide-loaded solid lipid nanoparticles for improved topical ocular delivery: tear kinetics and ocular disposition studies. Nanomaterials (Basel). 2018;9(1). https://doi.org/10.3390/nano9010033.
Chetoni P, Burgalassi S, Monti D, Tampucci S, Tullio V, Cuffini AM, et al. Solid lipid nanoparticles as promising tool for intraocular tobramycin delivery: pharmacokinetic studies on rabbits. Eur J Pharm Biopharm. 2016;109:214–23. https://doi.org/10.1016/j.ejpb.2016.10.006.
Balguri SP, Adelli GR, Majumdar S. Topical ophthalmic lipid nanoparticle formulations (SLN, NLC) of indomethacin for delivery to the posterior segment ocular tissues. Eur J Pharm Biopharm. 2016;109:224–35. https://doi.org/10.1016/j.ejpb.2016.10.015.
Aytekin E, Ozturk N, Vural I, Polat HK, Cakmak HB, Calis S, et al. Design of ocular drug delivery platforms and in vitro - in vivo evaluation of riboflavin to the cornea by non-interventional (epi-on) technique for keratoconus treatment. J Control Release. 2020;324:238–49. https://doi.org/10.1016/j.jconrel.2020.05.017.
Pai RV, Vavia PR. Chitosan oligosaccharide enhances binding of nanostructured lipid carriers to ocular mucins: effect on ocular disposition. Int J Pharm. 2020;577:119095. https://doi.org/10.1016/j.ijpharm.2020.119095.
Almeida H, Lobao P, Frigerio C, Fonseca J, Silva R, Sousa Lobo JM, et al. Preparation, characterization and biocompatibility studies of thermoresponsive eyedrops based on the combination of nanostructured lipid carriers (NLC) and the polymer Pluronic F-127 for controlled delivery of ibuprofen. Pharm Dev Technol. 2017;22(3):336–49. https://doi.org/10.3109/10837450.2015.1125922.
Ustundag-Okur N, Gokce EH, Bozbiyik DI, Egrilmez S, Ozer O, Ertan G. Preparation and in vitro-in vivo evaluation of ofloxacin loaded ophthalmic nano structured lipid carriers modified with chitosan oligosaccharide lactate for the treatment of bacterial keratitis. Eur J Pharm Sci. 2014;63:204–15. https://doi.org/10.1016/j.ejps.2014.07.013.
Tuomela A, Liu P, Puranen J, Ronkko S, Laaksonen T, Kalesnykas G, et al. Brinzolamide nanocrystal formulations for ophthalmic delivery: reduction of elevated intraocular pressure in vivo. Int J Pharm. 2014;467(1–2):34–41. https://doi.org/10.1016/j.ijpharm.2014.03.048.
Romero GB, Keck CM, Muller RH, Bou-Chacra NA. Development of cationic nanocrystals for ocular delivery. Eur J Pharm Biopharm. 2016;107:215–22. https://doi.org/10.1016/j.ejpb.2016.07.005.
Yandrapu SK, Kanujia P, Chalasani KB, Mangamoori L, Kolapalli RV, Chauhan A. Development and optimization of thiolated dendrimer as a viable mucoadhesive excipient for the controlled drug delivery: an acyclovir model formulation. Nanomed Nanotechnol Biol Med. 2013;9(4):514–22. https://doi.org/10.1016/j.nano.2012.10.005.
Tai L, Liu C, Jiang K, Chen X, Feng L, Pan W, et al. A novel penetratin-modified complex for noninvasive intraocular delivery of antisense oligonucleotides. Int J Pharm. 2017;529(1–2):347–56. https://doi.org/10.1016/j.ijpharm.2017.06.090.
Bachu RD, Chowdhury P, Al-Saedi ZHF, Karla PK, Boddu SHS. Ocular drug delivery barriers-role of nanocarriers in the treatment of anterior segment ocular diseases. Pharmaceutics. 2018;10(1). https://doi.org/10.3390/pharmaceutics10010028.
Reimondez-Troitino S, Csaba N, Alonso MJ, de la Fuente M. Nanotherapies for the treatment of ocular diseases. Eur J Pharm Biopharm. 2015;95(Pt B):279–93. https://doi.org/10.1016/j.ejpb.2015.02.019.
Lalu L, Tambe V, Pradhan D, Nayak K, Bagchi S, Maheshwari R, et al. Novel nanosystems for the treatment of ocular inflammation: current paradigms and future research directions. J Control Release. 2017;268:19–39. https://doi.org/10.1016/j.jconrel.2017.07.035.
Bodoki E, Vostinaru O, Samoila O, Dinte E, Bodoki AE, Swetledge S, et al. Topical nanodelivery system of lutein for the prevention of selenite-induced cataract. Nanomed Nanotechnol Biol Med. 2019;15(1):188–97. https://doi.org/10.1016/j.nano.2018.09.016.
Goyal R, Macri LK, Kaplan HM, Kohn J. Nanoparticles and nanofibers for topical drug delivery. J Control Release. 2016;240:77–92. https://doi.org/10.1016/j.jconrel.2015.10.049.
Yu Y, Feng R, Li J, Wang Y, Song Y, Tan G, et al. A hybrid genipin-crosslinked dual-sensitive hydrogel/nanostructured lipid carrier ocular drug delivery platform. Asian J Pharm Sci. 2019;14(4):423–34. https://doi.org/10.1016/j.ajps.2018.08.002.
Orasugh JT, Sarkar G, Saha NR, Das B, Bhattacharyya A, Das S, et al. Effect of cellulose nanocrystals on the performance of drug loaded in situ gelling thermo-responsive ophthalmic formulations. Int J Biol Macromol. 2019;124:235–45. https://doi.org/10.1016/j.ijbiomac.2018.11.217.
Lancina MG 3rd, Singh S, Kompella UB, Husain S, Yang H. Fast dissolving dendrimer nanofiber mats as alternative to eye drops for more efficient antiglaucoma drug delivery. ACS Biomater Sci Eng. 2017;3(8):1861–8. https://doi.org/10.1021/acsbiomaterials.7b00319.
Mishra V, Jain NK. Acetazolamide encapsulated dendritic nano-architectures for effective glaucoma management in rabbits. Int J Pharm. 2014;461(1–2):380–90. https://doi.org/10.1016/j.ijpharm.2013.11.043.
Holden CA, Tyagi P, Thakur A, Kadam R, Jadhav G, Kompella UB, et al. Polyamidoamine dendrimer hydrogel for enhanced delivery of antiglaucoma drugs. Nanomed Nanotechnol Biol Med. 2012;8(5):776–83. https://doi.org/10.1016/j.nano.2011.08.018.
Zuccari G, Carosio R, Fini A, Montaldo PG, Orienti I. Modified polyvinylalcohol for encapsulation of all-trans-retinoic acid in polymeric micelles. J Control Release. 2005;103(2):369–80. https://doi.org/10.1016/j.jconrel.2004.12.016.
Morsi N, Ibrahim M, Refai H, El Sorogy H. Nanoemulsion-based electrolyte triggered in situ gel for ocular delivery of acetazolamide. Eur J Pharm Sci. 2017;104:302–14. https://doi.org/10.1016/j.ejps.2017.04.013.
Ahmed S, Kassem MA, Sayed S. Bilosomes as promising nanovesicular carriers for improved transdermal delivery: construction, in vitro optimization, ex vivo permeation and in vivo evaluation. Int J Nanomed. 2020;15:9783–98. https://doi.org/10.2147/IJN.S278688.
Ahmed S, Kassem MA, Sayed S. Co-polymer mixed micelles enhanced transdermal transport of lornoxicam: in vitro characterization, and in vivo assessment of anti-inflammatory effect and antinociceptive activity. J Drug Deliv Sci Technol. 2021;62:102365. https://doi.org/10.1016/j.jddst.2021.102365.
Mosallam S, Ragaie MH, Moftah NH, Elshafeey AH, Abdelbary AA. Use of novasomes as a vesicular carrier for improving the topical delivery of terconazole: in vitro characterization, in vivo assessment and exploratory clinical experimentation. Int J Nanomed. 2021;16:119–32. https://doi.org/10.2147/IJN.S287383.
Daull P, Buggage R, Lambert G, Faure MO, Serle J, Wang RF, et al. A comparative study of a preservative-free latanoprost cationic emulsion (Catioprost) and a BAK-preserved latanoprost solution in animal models. J Ocul Pharmacol Ther. 2012;28(5):515–23. https://doi.org/10.1089/jop.2011.0245.
Jurisic Dukovski B, Juretic M, Bracko D, Randjelovic D, Savic S, Crespo Moral M, et al. Functional ibuprofen-loaded cationic nanoemulsion: development and optimization for dry eye disease treatment. Int J Pharm. 2020;576:118979. https://doi.org/10.1016/j.ijpharm.2019.118979.
Fialho SL, da Silva-Cunha A. New vehicle based on a microemulsion for topical ocular administration of dexamethasone. Clin Exp Ophthalmol. 2004;32(6):626–32. https://doi.org/10.1111/j.1442-9071.2004.00914.x.
Ismail A, Nasr M, Sammour O. Nanoemulsion as a feasible and biocompatible carrier for ocular delivery of travoprost: improved pharmacokinetic/pharmacodynamic properties. Int J Pharm. 2020;583:119402. https://doi.org/10.1016/j.ijpharm.2020.119402.
Kumar S, Karki R, Meena M, Prakash T, Rajeswari T, Goli D. Reduction in drop size of ophthalmic topical drop preparations and the impact of treatment. J Adv Pharm Technol Res. 2011;2(3):192–4. https://doi.org/10.4103/2231-4040.85540.
Hotujac Grgurevic M, Juretic M, Hafner A, Lovric J, Pepic I. Tear fluid-eye drops compatibility assessment using surface tension. Drug Dev Ind Pharm. 2017;43(2):275–82. https://doi.org/10.1080/03639045.2016.1238924.
Shen S, Wu Y, Liu Y, Wu D. High drug-loading nanomedicines: progress, current status, and prospects. Int J Nanomed. 2017;12:4085–109. https://doi.org/10.2147/IJN.S132780.
Lin HL, Cheng WT, Chen LC, Ho HO, Lin SY, Hsieh CM. Honokiol/magnolol-loaded self-assembling lecithin-based mixed polymeric micelles (lbMPMs) for improving solubility to enhance oral bioavailability. Int J Nanomed. 2021;16:651–65. https://doi.org/10.2147/IJN.S290444.
Yamaguchi M, Ueda K, Isowaki A, Ohtori A, Takeuchi H, Ohguro N, et al. Mucoadhesive properties of chitosan-coated ophthalmic lipid emulsion containing indomethacin in tear fluid. Biol Pharm Bull. 2009;32(7):1266–71. https://doi.org/10.1248/bpb.32.1266.
Gonzalez-Mira E, Egea MA, Garcia ML, Souto EB. Design and ocular tolerance of flurbiprofen loaded ultrasound-engineered NLC. Colloids Surf, B. 2010;81(2):412–21. https://doi.org/10.1016/j.colsurfb.2010.07.029.
Mahboobian MM, Mohammadi M, Mansouri Z. Development of thermosensitive in situ gel nanoemulsions for ocular delivery of acyclovir. J Drug Deliv Sci Technol. 2020;55:101400. https://doi.org/10.1016/j.jddst.2019.101400.
Yousry C, Elkheshen SA, El-Laithy HM, Essam T, Fahmy RH. Studying the influence of formulation and process variables on vancomycin-loaded polymeric nanoparticles as potential carrier for enhanced ophthalmic delivery. Eur J Pharm Sci. 2017;100:142–54. https://doi.org/10.1016/j.ejps.2017.01.013.
Moiseev RV, Morrison PWJ, Steele F, Khutoryanskiy VV. Penetration enhancers in ocular drug delivery. Pharmaceutics. 2019;11(7). https://doi.org/10.3390/pharmaceutics11070321.
Li Y, Zhang Y, Li P, Mi G, Tu J, Sun L, et al. Ion-paired pirenzepine-loaded micelles as an ophthalmic delivery system for the treatment of myopia. Nanomed Nanotechnol Biol Med. 2017;13(6):2079–89. https://doi.org/10.1016/j.nano.2017.05.001.
Ye T, Yuan K, Zhang W, Song S, Chen F, Yang X, et al. Prodrugs incorporated into nanotechnology-based drug delivery systems for possible improvement in bioavailability of ocular drugs delivery. Asian J Pharm Sci. 2013;8(4):207–17. https://doi.org/10.1016/j.ajps.2013.09.002.
Grassiri B, Zambito Y, Bernkop-Schnurch A. Strategies to prolong the residence time of drug delivery systems on ocular surface. Adv Colloid Interface Sci. 2021;288:102342. https://doi.org/10.1016/j.cis.2020.102342.
Ding H, Shu X, Jin Y, Fan T, Zhang H. Recent advances in nanomaterial-enabled acoustic devices for audible sound generation and detection. Nanoscale. 2019;11(13):5839–60. https://doi.org/10.1039/c8nr09736d.
Xie Z, Chen S, Duo Y, Zhu Y, Fan T, Zou Q, et al. Biocompatible two-dimensional titanium nanosheets for multimodal imaging-guided cancer theranostics. ACS Appl Mater Interfaces. 2019;11(25):22129–40. https://doi.org/10.1021/acsami.9b04628.
Tsujinaka H, Fu J, Shen J, Yu Y, Hafiz Z, Kays J, et al. Sustained treatment of retinal vascular diseases with self-aggregating sunitinib microparticles. Nat Commun. 2020;11(1):694. https://doi.org/10.1038/s41467-020-14340-x.
Del Pozo-Rodriguez A, Solinis MA, Rodriguez-Gascon A. Applications of lipid nanoparticles in gene therapy. Eur J Pharm Biopharm. 2016;109:184–93. https://doi.org/10.1016/j.ejpb.2016.10.016.
Basuki JS, Qie F, Mulet X, Suryadinata R, Vashi AV, Peng YY, et al. Photo-modulated therapeutic protein release from a hydrogel depot using visible light. Angew Chem Int Ed Engl. 2017;56(4):966–71. https://doi.org/10.1002/anie.201610618.
Li N, Zhao L, Wei Y, Ea VL, Nian H, Wei R. Recent advances of exosomes in immune-mediated eye diseases. Stem Cell Res Ther. 2019;10(1):278. https://doi.org/10.1186/s13287-019-1372-0.
Tang Q, Lu B, He J, Chen X, Fu Q, Han H, et al. Exosomes-loaded thermosensitive hydrogels for corneal epithelium and stroma regeneration. Biomaterials. 2022;280:121320. https://doi.org/10.1016/j.biomaterials.2021.121320.
Zhu S, Huang H, Liu D, Wen S, Shen L, Lin Q. Augmented cellular uptake and homologous targeting of exosome-based drug loaded IOL for posterior capsular opacification prevention and biosafety improvement. Bioact Mater. 2022. https://doi.org/10.1016/j.bioactmat.2022.02.019.
Ellis-Behnke RG, Liang YX, You SW, Tay DK, Zhang S, So KF, et al. Nano neuro knitting: peptide nanofiber scaffold for brain repair and axon regeneration with functional return of vision. Proc Natl Acad Sci U S A. 2006;103(13):5054–9. https://doi.org/10.1073/pnas.0600559103.
Ellis-Behnke R, Jonas JB. Redefining tissue engineering for nanomedicine in ophthalmology. Acta Ophthalmol. 2011;89(2):e108–14. https://doi.org/10.1111/j.1755-3768.2010.01982.x.
Lee J, Boo C, Ryu WH, Taylor AD, Elimelech M. Development of omniphobic desalination membranes using a charged electrospun nanofiber scaffold. ACS Appl Mater Interfaces. 2016;8(17):11154–61. https://doi.org/10.1021/acsami.6b02419.
Ellenberg D, Shi J, Jain S, Chang JH, Ripps H, Brady S, et al. Impediments to eye transplantation: ocular viability following optic-nerve transection or enucleation. Br J Ophthalmol. 2009;93(9):1134–40. https://doi.org/10.1136/bjo.2008.155267.
Kalishwaralal K, Barathmanikanth S, Pandian SR, Deepak V, Gurunathan S. Silver nano - a trove for retinal therapies. J Control Release. 2010;145(2):76–90. https://doi.org/10.1016/j.jconrel.2010.03.022.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Funding Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Conceptualization: S.A., M.M.A., and S.S.; software: S.A.; formal analysis: S.A., M.M.A., and S.S.; investigation: S.A., M.M.A., and S.S.; resources: S.A, M.M.A, and S.S.; writing—original draft preparation: S.A.; writing—review and editing: M.M.A. and S.S.; supervision: M.M.A. and S.S. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ahmed, S., Amin, M.M. & Sayed, S. Ocular Drug Delivery: a Comprehensive Review. AAPS PharmSciTech 24, 66 (2023). https://doi.org/10.1208/s12249-023-02516-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02516-9