Abstract
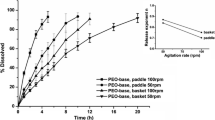
The physiologically relevant dissolution apparatuses simulate various aspects of gastrointestinal physiology and help to understand and predict the in vivo behavior of an oral dosage form. In this paper, we present and characterize for the first time a novel bio-relevant dissolution apparatus — PhysioCell. We evaluated the impact of several factors on the hydrodynamic conditions in the key vessel of the apparatus — the StressCell. We observed that the medium flow rate, but not the glass beads’ size or amount, significantly influenced the dissolution rate. The relationship was disproportional: the increase in the flow rate from 4.6 to 9.0 mL/min reduced the dissolution time of 85% (T85) of the NaCl tablet by 46%, but from 134 to 300 mL/min decreased the T85 only by 24%. At the same time, the contractions of the StressCell’s elastic walls promoted the content mixing and enhanced the dissolution rate of the paracetamol tablets: even very rare mixing contractions (1 per 10 min) decreased the T85 over twofold for the flow rate of 8 mL/min. In conclusion, the hydrodynamic conditions in the StressCell affect the dissolution of solid dosage forms and the understanding of these effects is crucial for modeling physiologically-based test conditions in the novel apparatus. Combinations of the unique PhysioCell features — adjustable medium flow, temperature control, controllable pH gradients and predefined mechanical agitation — can create a set of dissolution test scenarios for characterization of oral dosage forms and, in the future, making the in vitro-in vivo predictions.

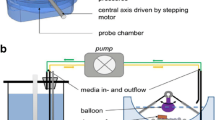
Visualisation of the operating parameters of the PhysioCell that were examined in this study.







Similar content being viewed by others
Change history
21 March 2023
A Correction to this paper has been published: https://doi.org/10.1208/s12249-023-02543-6
References
Garbacz G, Rappen GM, Koziolek M, Weitschies W. Dissolution of mesalazine modified release tablets under standard and bio-relevant test conditions. J Pharm Pharm 2015;67(2):199–208.
Fadda HM, Merchant HA, Arafat BT, Basit AW. Physiological bicarbonate buffers: stabilisation and use as dissolution media for modified release systems. Int J Pharm 2009;382(1-2):56– 60.
Jantratid E, De Maio V, Ronda E, Mattavelli V, Vertzoni M, Dressman JB. Application of biorelevant dissolution tests to the prediction of in vivo performance of diclofenac sodium from an oral modified-release pellet dosage form. Eur J Pharm Sci 2009;37(3-4):434–441.
Schiller C, Fröhlich CP, Giessmann T, Siegmund W, Mönnikes H, Hosten N, et al. Intestinal fluid volumes and transit of dosage forms as assessed by magnetic resonance imaging. Alimentary Pharmacology & Therapeutics 2005;22:971–979.
Schneider F, Grimm M, Koziolek M, Modeß C, Dokter A, Roustom T, et al. Resolving the physiological conditions in bioavailability and bioequivalence studies: Comparison of fasted and fed state. Eur J Pharm Biopharm 2016;108:214–219.
Garbacz G, Cadé D, Benameur H, Weitschies W. Bio-relevant dissolution testing of hard capsules prepared from different shell materials using the dynamic open flow through test apparatus. European J Pharm Sci 2014;57:264–272.
Jede C, Wagner C, Kubas H, Weigandt M, Weber C, Lecomte M, et al. Improved prediction of in vivo supersaturation and precipitation of poorly soluble weakly basic drugs using a biorelevant bicarbonate buffer in a gastrointestinal transfer model. Molecular Pharmaceutics 2019;16(9):3938–3947.
Garbacz G, Wedemeyer RS, Nagel S, Giessmann T, Monnikes H, Wilson CG, et al. Irregular absorption profiles observed from diclofenac extended release tablets can be predicted using a dissolution test apparatus that mimics in vivo physical stresses. European Journal of Pharmaceutics and Biopharmaceutics 2008; 70:421–428.
Diebold S. 2005. Physiological parameters relevant to dissolution testing: hydrodynamic considerations. Taylor & Francis: London UK.
Abrahamsson A, Pal M, Sjoberg M, Carlsson E, Laurell JGB. A novel in vitro and numerical analysis of shear-induced drug release from extended-release tablets in the fed stomach. Pharmaceutical Research 2005;22(8):1215–1226.
Wiater M, Hoc D, Paszkowska J, Garbacz G. Hydrodynamics and mechanical stresses in pharmacopoeial and noncompendial dissolution testing. Farmacja Polska 2020;76(4):210–221.
McAllister M. Dynamic dissolution: a step closer to predictive dissolution testing?. Molecular Pharmaceutics 2010;7(5):1374–1387.
Minekus M. The TNO gastro-intestinal model (TIM). Cham: Springer; 2015, pp. 37–46.
Hribar M, Trontelj J, Berglez S, Bevc A, Kuscer L, Diaci J, et al. 2019. Design of an innovative advanced gastric simulator. Dissolution Technologies, 20–29.
Wickham MSJ, Faulks RM, Mann J, Mandalari G. The design, operation, and application of a dynamic gastric model. Dissolution Technologies 2012;19(3):15–22.
Kourentas A, Vertzoni M, Stavrinoudakis N, Symillidis A, Brouwers J, Augustijns P, et al. An in vitro biorelevant gastrointestinal transfer (BioGIT) system for forecasting concentrations in the fasted upper small intestine: design, implementation, and evaluation. European Journal of Pharmaceutical Sciences 2016; 82:106–114.
Schick P, Sager M, Wegner F, Wiedmann M, Schapperer E, Weitschies W, et al. Application of the GastroDuo as an in vitro dissolution tool to simulate the gastric emptying of the postprandial stomach. Molecular Pharmaceutics 2019;16(11):4651– 4660.
Li Y, Kong F. Simulating human gastrointestinal motility in dynamic in vitro models. Compr Rev Food Sci Food Safety 2022;21(5):3804–3833.
Wilson C, Aarons L, Augustijns P, Brouwers J, Darwich A, De Waal T, et al. 2021. Integration of advanced methods and models to study drug absorption and related processes: An UNGAP perspective. European Journal of Pharmaceutical Sciences, 106100.
Staniszewska M, Kolodziej B, Paszkowska J, Dobosz J, Garbacz G, Banach G. 2021. A flow-through cell for testing the behavior of a pharmaceutical dosage form, the system containing it and the method of homogenizing the dissolution medium P.43946.
Koziolek M, Schneider F, Grimm M, Modess C, Seekamp A, Roustom T, et al. Intragastric pH and pressure profiles after intake of the high-caloric, high-fat meal as used for food effect studies. J Controlled Release 2015;220:71–78.
Koziolek M, Grimm M, Becker D. Investigation of pH and Temperature Profiles in the GI Tract of Fasted Human Subjects Using the Intellicap System. Pharmaceutics, Drug Del Pharm Technol 2014;104: 2855–2863.
Shiko G, Gladden L, Sederman A, Connolly P, Butler J. MRI studies of the hydrodynamics in a USP 4 dissolution testing cell. J Pharmaceutical Sci 2011;100(3):976–991.
Eaton J W, Tran D, Hauck W W, Stippler ES. Development of a performance verification test for USP apparatus 4. Pharmaceutical Research 2012;29(2):345–351.
Krämer J, Stippler E. Experiences with USP apparatus 4 calibration. Dissolution Technol 2005; 12(2):33–39.
Fang J, Robertson V, Rawat A, Flick T, Tang Z, Cauchon N, et al. Development and application of a biorelevant dissolution method using USP apparatus 4 in early phase formulation development. Molecular Pharmaceutics 2010;7(5):1466–1477.
D’Arcy D, Corrigan O, Healy AM. Evaluation of hydrodynamics in the basket dissolution apparatus using computational fluid dynamics—dissolution rate implications. European J Pharmaceutical Sci 2006;27(2-3): 259–267.
Stevens L, Missel P. Impact of density gradients on flow-through dissolution in a cylindrical flow cell. Pharm Dev Technol 2006;11(4):529–534.
McDonnell D, D’Arcy D, Crane L, Redmond B. A mathematical analysis of drug dissolution in the USP flow through apparatus. Heat and Mass Transfer 2018;54(3):793–801.
D’Arcy D, Liu B, Corrigan O. Investigating the effect of solubility and density gradients on local hydrodynamics and drug dissolution in the USP 4 dissolution apparatus. Int J Pharm 2011;419(1-2): 175–185.
Missel PJ, Stevens LE, Mauger JW. Reexamination of convective diffusion/drug dissolution in a laminar flow channel: accurate prediction of dissolution rate. Pharm Res 2004;21(12):2300–2306.
Sleziona D, Mattusch A, Schaldach G, Ely D R, Sadowski G, Thommes M. Determination of inherent dissolution performance of drug substances. Pharmaceutics 2021;13(2):146.
Marciani L, Gowland PA, Spiller RC, Manoj P, Moore RJ, Young P, et al. Effect of meal viscosity and nutrients on satiety, intragastric dilution, and emptying assessed by MRI. Am J Physiol-Gastrointestinal Liver Physiol 2001;280(6):G1227–G1233.
Mudie D M, Murray K, Hoad CL, Pritchard SE, Garnett MC, Amidon GL, et al. Quantification of gastrointestinal liquid volumes and distribution following a 240 mL dose of water in the fasted state. Mol Pharmaceutics 2014;11(9):3039–3047.
Steingoetter A, Fox M, Treier R, Weishaupt D, Marincek B, Boesiger P, et al. Effects of posture on the physiology of gastric emptying: a magnetic resonance imaging study. Scand J Gastroenterol 2006; 41(10):1155–1164.
Goyal R K, Guo Y, Mashimo H. Advances in the physiology of gastric emptying. Neurogastroenterology & Motility 2019;31(4):e13546.
Weitschies W, Blume H, Mönnikes H. Magnetic marker monitoring: high resolution real-time tracking of oral solid dosage forms in the gastrointestinal tract. Eur J Pharm Biopharm 2010;74(1):93–101.
Schiller C, Fröhlich C P, Giessmann T, Siegmund W, Mönnikes H, Hosten N, et al. Intestinal fluid volumes and transit of dosage forms as assessed by magnetic resonance imaging. Aliment Pharmacol Ther 2005;22(10):971–979.
Acknowledgements
The authors would like to kindly thank Prof. Sebastian Polak, PhD Grzegorz Banach and PhD Jadwiga Paszkowska for critically reviewing the final version of the manuscript.
Funding
The work has been partially financed by Polish National Centre for Research and Development (POIR.01.02.00-00-0011/17).
Author information
Authors and Affiliations
Contributions
Conceptualization: Marcela Staniszewska, Grzegorz Garbacz. Methodology: Marcela Staniszewska, Justyna Dobosz, Bartosz Kołodziej, Uladzimir Lipski. Formal analysis and investigation: Marcela Staniszewska, Grzegorz Garbacz. Writing — original draft preparation: Marcela Staniszewska, Dorota Danielak. Writing — review and editing: Michał; Romański, Grzegorz Garbacz. Funding acquisition: Grzegorz Garbacz. Supervision: Dorota Danielak, Grzegorz Garbacz.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original article has been updated to correct Michał Romański and Bartosz Kołodziej names by including Polish characters. Previously published article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Staniszewska, M., Romański, M., Dobosz, J. et al. PhysioCell — a Novel, Bio-relevant Dissolution Apparatus: Hydrodynamic Conditions and Factors Influencing the Dissolution Dynamics. AAPS PharmSciTech 24, 65 (2023). https://doi.org/10.1208/s12249-022-02494-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02494-4