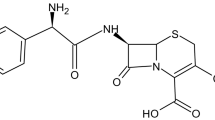
Abstract
For the purpose of establishing the optimum processing parameters and storage conditions associated with nanolipid formulations of the artemisinin derivative artesunate, it was necessary to evaluate the thermal stability and solubility profiles of artesunate in aqueous solutions at various temperatures and pH. The effect of increased temperature and humidity on artesunate was determined by storing samples of the raw material in a climate chamber for 3 months and analyzing these by an established HPLC method. Artesunate remained relatively stable during storage up to 40°C ± 0.5°C and 75% relative humidity for 3 months, wherein it undergoes approximately 9% decomposition. At higher temperatures, substantially greater decomposition supervenes, with formation of dihydroartemisinin (DHA) and other products. In solution, artesunate is relatively stable at 15°C with less than 10% degradation over 24 h. The aqueous solubility of artesunate at different pH values after 60 min are pH 1.2 (0.1 M HCl) 0.26 mg/mL, pH 4.5 (acetate buffer) 0.92 mg/mL, distilled water 1.40 mg/mL, and pH 6.8 (phosphate buffer) 6.59 mg/mL, thus relating to the amount of ionized drug present. Overall, for optimal preparation and storage of the designated formulations of artesunate, relatively low temperatures will have to be maintained throughout.




Similar content being viewed by others
Data Availability
All data generated during this study is available upon request to the authors.
References
Hsu E. Reflections on the ‘discovery’ of the antimalarial qinghao. Br J Clin Pharmacol. 2006;61:666–70. https://doi.org/10.1111/j.1365-2125.2006.02673.x.
Cui L, Su XZ. Discovery, mechanisms of action and combination therapy of artemisinin. Expert Rev Anti Infect Ther. 2009;7:999–1003. https://doi.org/10.1586/eri.09.68.
Guoqiao L, Ying L, Zelin L, Meiyi Z. Abstract. In: Guoqiao L, Ying L, Zelin L, Meiyi Z. Cambridge, editors. Artemisinin-based and other antimalarials: detailed account of studies by Chinese scientists who discovered and developed them. Academic Press, Elsevier; 2017 p. 1–67.
Tiwari MK, Chaudhary S. Artemisinin‐derived antimalarial endoperoxides from bench‐side to bed‐side: chronological advancements and future challenges. Med Res Rev. 2020; 1–56. https://doi.org/10.1002/med.21657.
Ho WE, Peh HY, Chan TK, Wong WSF. Artemisinins: pharmacological actions beyond anti-malarial. Pharmacol Ther. 2014;142:126e139. https://doi.org/10.1016/j.pharmthera.2013.12.001.
Loo CSN, Lam NSK, Yu D, Su X-Z, Lu F. Artemisinin and its derivatives in treating protozoan infections beyond malaria. Pharmacol Res. 2017;117:192–217. https://doi.org/10.1016/j.phrs.2016.11.012.
Kiani BH, Kayani WK, Khayam AU, Dilshad E, Ismail H, Mirza B. Artemisinin and its derivatives: a promising cancer therapy. Mol Biol Rep. 2020;47:6321–36. https://doi.org/10.1007/s11033-020-05669-z.
Xu C, Zhang H, Mu L, Yang. Artemisinins as anticancer drugs: novel therapeutic approaches, molecular mechanisms, and clinical trials. Front Pharmacol. 2020;11:529881. https://doi.org/10.3389/fphar.2020.529881.
Dwivedi A, Mazumder A, Du Plessis L, Du Preez JL, Haynes RK, Du Plessis J. In vitro anti-cancer effects of artemisone nano-vesicular formulations on melanoma cells. Nanomedicine: Nanotechnology. Biol Med. 2015;11:2041–50. https://doi.org/10.1016/j.nano.2015.07.010.
Burger C, Aucamp M, Du Preez J, Haynes RK, Ngwane A, Du Plessis J, et al. Formulation of natural oil nano-emulsions for the topical delivery of clofazimine, artemisone and decoquinate. Pharm Res. 2018;35:186. https://doi.org/10.1007/s11095-018-2471-9.
Chadha R, Gupta S, Pathak N. Artesunate-loaded chitosan/lecithin nanoparticles: preparation, characterization, and in vivo studies. Drug Dev Ind Pharm. 2012;38(12):1538–46. https://doi.org/10.3109/03639045.2012.658812.
Batty KT, Ilett KF, Davis TME. Chemical stability of artesunate injection and proposal for its administration by intravenous infusion. J Pharm Pharmacol. 1996;48:22–6. https://doi.org/10.1111/j.2042-7158.1996.tb05870.x.
Hendriksen ICE, Mtove G, Kent A, Gesase S, Reyburn H, Lemnge MM, et al. Population pharmacokinetics of intramuscular artesunate in African children with severe malaria: implications for a practical dosing regimen. Clin Pharmacol Therapeutics. 2013;93:443–50. https://doi.org/10.1038/clpt.2013.26.
Anon. Guidelines for administration of injectable artesunate for severe malaria. 2014. https://www.mmv.org/sites/default/files/uploads/docs/access/Injectable_Artesunate_Tool_Kit/InjectableArtesunate_posterEN.pdf. Accessed 22 Mar 2022.
Newton P, Supittamongkol Y, Teja-Isavadharm P, Pukrittayakamee S, Navaratnam V, Bates I, et al. Antimalarial bioavailability and disposition of artesunate in acute falciparum malaria. Antimicrob Agents Chemother. 2000;4:972–7. https://doi.org/10.1128/AAC.44.4.972-977.2000.
Morris CA, Duparc S, Borghini-Fuhrer I, Jung D, Shin CS, Fleckenstein L. Review of the clinical pharmacokinetics of artesunate and its active metabolite dihydroartemisinin following intravenous, intramuscular, oral or rectal administration. Malar J. 2011;10:263. https://doi.org/10.1186/1475-2875-10-263.
Haynes RK, Chan HW, Cheung MK, Lam WL, Soo MK, Tsang HW, et al. ID. C-10 ester and ether derivatives of dihydroartemisinin: 10-α artesunate, preparation of authentic 10-β artesunate, and of other esters and ether derivatives bearing potential aromatic intercalating groups at C-10. Eur J Org Chem. 2002; 113–132. https://doi.org/10.1002/1099-0690(20021)2002:1<113::AID-EJOC113>3.0.CO;2-N.
Batty KT, Ilett KE, Powell SM, Martin J, Davis TME. Relative bioavailability of artesunate and dihydroartemisinin: investigations in the isolated perfused rat liver and in healthy Caucasian volunteers. Am J Trop Med Hyg. 2002;66:130–6. https://doi.org/10.4269/ajtmh.2002.66.130.
Agnihotri J, Singh S, Bigonia P. Formal chemical stability analysis and solubility analysis of artesunate and hydroxychloroquine for development of parenteral dosage form. J Pharm Res. 2013;6:117–22. https://doi.org/10.1016/j.jopr.2012.11.025.
Haynes RK, Chan HW, Lung CM, Ng NC, Wong HN, Shek LY, et al. Artesunate and dihydroartemisinin (DHA): unusual decomposition products formed under mild conditions and comments on the fitness of DHA as an antimalarial drug. ChemMedChem. 2007;2:1448–63. https://doi.org/10.1002/cmdc.200700064.
Lin AJ, Lee M, Klayman DL. Antimalarial activity of new water-soluble dihydroartemisinin derivatives. 2. Stereospecificity of the ether side chain. J Med Chem. 1989;32:1249–52. https://doi.org/10.1021/jm00126a017.
Ashford M. Bioavailability – physicochemical and dosage form factors. In: Aulton ME, Taylor KMG, editors. Aulton’s pharmaceutics: the design and manufacture of medicines. Amsterdam: Elsevier; 2013. p. 314–33.
Li Y. Qinghaosu (artemisinin): chemistry and pharmacology. Acta Pharmacol Sin. 2012;33:1141–6. https://doi.org/10.1038/aps.2012.104.
Haynes RK. From artemisinin to new artemisinin antimalarials: biosynthesis, extraction, old and new derivatives, stereochemistry and medicinal chemistry requirements. Curr Top Med Chem. 2006;6:509–37. https://doi.org/10.2174/156802606776743129.
Watson DJ, Laing L, Gibhard L, Wong HN, Haynes RK, Wiesner L. Towards new transmission-blocking combination therapies - pharmacokinetics of 10-amino-artemisinins and 11-aza-artemisinin, and comparison with dihydroartemisinin and artemether. Antimicrob Agents Chemother. 2021;65:e00990-e1021. https://doi.org/10.1128/AAC.00990-21.
Pubchem, NIH US National Library of Medicine, National Center for Biotechnology Information. https://pubchem.ncbi.nlm.nih.gov/compound/Artesunic-acid (Accessed 13 May 2022).
Tran TH, Nguyen TD, Poudel BK, Nguyen HT, Kim JO, Yong CS, et al. Development and evaluation of artesunate-loaded chitosan-coated lipid nanocapsule as a potential drug delivery system against breast cancer. AAPS PharmSciTech. 2015;16:1307–16. https://doi.org/10.1208/s12249-015-0311-3.
Xu R, Han T, Shen L, Zhao J, Lu X. Solubility determination and modelling for artesunate in binary solvent mixtures of methanol, ethanol, isopropanol and propylene glycol and water. J Chem Eng Data. 2019;64:755–62. https://doi.org/10.1021/acs.jced.8b00988.
Lin AJ, Klayman DL, Milhous WK. Antimalarial activity of new water-soluble dihydroartemisinin derivatives. J Med Chem. 1987;30:2147–50. https://doi.org/10.1021/jm00394a037.
Luo XD, Yeh HJC, Brossi A, Flippen-Anderson JL. Gillardi R The chemistry of drugs part IV: configurations of antimalarials derived from qinghaosu: dihydroqinghaosu, artemether, and artesunic acid. Helv Chim Acta. 1984;67:1515–22. https://doi.org/10.1002/hlca.19840670615.
Batty KT, Ilett KF, Davis TME. Protein binding and α:β anomer ratio of dihydroartemisinin in vivo. Br J Clin Pharmacol. 2004;57:529–33. https://doi.org/10.1111/j.1365-2125.2004.02045.x.
Cabri W, ID’Acquarica I, Simone P, Di Iorio M, Di Mattia M Gasparrini F, et al. Stereolability of dihydroartemisinin, an antimalarial drug a comprehensive kinetic investigation. Part 2 J Org Chem. 2011;76:4831–40. https://doi.org/10.1021/jo102392p.
The International Pharmacopoeia 10th Edition 2020; Monographs, pharmaceutical substances. https://digicollections.net/phint/2020/index.html#d/b.6.1.30 (accessed 13 May 2022).
European Medicines Agency. Stability testing of new drug substances and products Q1A(R2); Q1A(R2) Stability Testing of New Drug Substances and Products. In: Guidance Document: International Conference on Harmonisation. International Conference on Harmonisation. https://www.ema.europa.eu/en/ich-q1a-r2-stability-testing-new-drug-substances-drug-products-scientific-guideline. Accessed 22 Mar 2022.
The International Pharmacopoeia 10th Edition 2021. https://www.gmp-compliance.org/gmp-news/10th-edition-of-the-international-pharmacopoeia. Accessed 13 May 2022.
Funding
This work was funded by the South African Medical Research Council (MRC) Flagship Project MALTB-Redox with funds from the National Treasury under its Economic Competitiveness and Support Package (UID MRC-RFA-UFSP-01–2013) (RKH) and by a South African National Research Foundation (SA NRF) grant (UID 129135) (RKH). The authors are grateful for the financial support of the Centre of Excellence for Pharmaceutical Sciences (Pharmacen) at the North-West University, Potchefstroom, South Africa.
Author information
Authors and Affiliations
Contributions
Post-graduate who was responsible for a substantial contribution to most of the analytical work: Bezuidenhout JW.
Conceptualization of the project: Liebenberg W, Aucamp M, and Stieger N.
Drafting and revising it critically for important intellectual content: Haynes RK and Aucamp M.
Final approval of the version to be published: Haynes RK, Liebenberg W, and Aucamp M.
All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Disclaimer
Any opinions, findings and conclusions, or recommendations expressed in this material are those of the authors and therefore the NRF does not accept any liability in regard thereto.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bezuidenhout, J.W., Aucamp, M., Stieger, N. et al. Assessment of Thermal and Hydrolytic Stabilities and Aqueous Solubility of Artesunate for Formulation Studies. AAPS PharmSciTech 24, 33 (2023). https://doi.org/10.1208/s12249-022-02490-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02490-8