Abstract
The aim of this study was to develop microemulsion (ME) formulation with possible phase transition into liquid crystals upon ocular application to enhance acetazolamide bioavailability. Pseudoternary phase diagrams were constructed using olive oil or castor oil (oily phase), Tween 80 (surfactant), and sodium carbonate solution (aqueous phase). Microemulsion and liquid crystal (LC) formulations were selected from the constructed phase diagrams and were evaluated for rheological properties and in vitro drug release. The efficacy of the developed formulations in reducing intraocular pressure (IOP) was assessed in vivo. In vitro release study showed slower release rate from LC and ME compared with drug solution with the release from LC being the slowest. Ocular application of acetazolamide ME formulations or aqueous solution resulted in significant reduction in IOP from baseline. The recorded Tmax values indicated faster onset of action for acetazolamide aqueous solution (1 h) compared with ME systems (3 h). However, the duration of action was prolonged and the reduction in IOP continued for up to 10 h in case of MEs, while that of aqueous solution was only for 4–5 h. The study suggested ME formulations for ocular delivery of acetazolamide with enhanced efficacy and prolonged duration of action.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glaucoma is a chronic and serious ocular disorder. The seriousness of glaucoma can be attributed to the pathophysiological changes that occur in ocular structure including the loss of retinal ganglion cells and thinning of the retinal nerve fiber layer. The problem would be aggravated to complete loss of vision in absence of proper glaucoma treatment. These changes are usually accompanied with an elevation in the intraocular pressure (IOP) which is the only curable symptoms in the pathophysiology of glaucoma [1].
Several active drug substances were utilized for treatment of glaucoma. Acetazolamide (AZA) is one of the active agents that showed noticeable success in glaucoma treatment after oral administration. It is a carbonic anhydrase inhibitor (CAIs) which can reduce elevated IOP and it was reported to be superior compared to other CAIs including brinzolamide, methazolamide, and dorzolamide in reducing IOP [2]. However, its efficiency necessitates the use of large oral doses to achieve the desired reduction in IOP. These large oral doses can lead to deleterious systemic complications, including renal toxicity, acidosis, and in some cases severe blood dyscrasias. Accordingly, ocular administration of the drug can reduce these serious systemic adverse effects. Unfortunately, acetazolamide is poorly water-soluble drug (0.7 mg/mL) with low corneal permeability which resulted in poor ocular bioavailability. So, no topical formulation of acetazolamide is available in markets till now [3,4,5].
In addition to the undesirable physicochemical properties of acetazolamide which hinder its ocular delivery, the anatomy and physiology of the eye represents another obstacle against ocular drug delivery. This is accredited to the rapid precorneal loss of the applied drug due to fast tear fluid turnover rate. The protective structure of the corneal membrane contributes significantly to the barrier for drug diffusion [6, 7]. Several strategies were conducted for enhancing ocular delivery of drugs. These include formulation of colloidal carriers and micellar systems [8,9,10,11,12], bioadhesive hydrogels [13, 14], in situ gel forming systems [15,16,17,18], and medicated contact lenses [19,20,21].
Microemulsions were reported as fruitful delivery systems for enhancing ocular delivery of many drugs [22,23,24,25,26]. A microemulsion (ME) can be fabricated by proper mixing of oily phase with surfactant and aqueous phase. Co-surfactants and electrolytes may also be incorporated into ME. ME has the advantages of spontaneous formation, thermodynamic stability, and greater solubilizing power for hydrophobic drugs [27, 28]. Upon increasing water concentration, ME can undergo phase transition to liquid crystalline, gel, or coarse emulsion phases. Phase transition is accompanied by alteration in rheological behavior with system thickening followed by thinning. This characteristic can be utilized for enhancing ocular contact time. This is based on the ability of liquid ME to undergo phase transition upon mixing with tear film to form a system with higher viscosity [24,25,26,27,28,29,30,31].
Accordingly, the aim of this work was to develop and optimize ME formulation for enhanced ocular delivery of acetazolamide after topical application. The work was extended to investigate the effect of the optimized formulations on the IOP in vivo. The goal was to control the intraocular pressure after topical application to avoid the side effects encountered after oral administration of acetazolamide.
Material and Methods
Materials
Acetazolamide was donated by Chemical Industries Development (CID). Castor oil, olive oil, sorbitan mono laurate (Span 20), polyoxy-ethylene 20 sorbitan mono-oleate (Tween 80), sodium bicarbonate, calcium chloride, potassium chloride, and sodium carbonate were obtained from El Nasr Pharmaceutical Chemicals Company, Cairo, Egypt. Methanol was obtained from Alpha Chemika Pharmaceutical Chemicals Company, Cairo, Egypt. Dialysis tubing cellulose membrane (molecular weight cutoff 12,000 g/mol) was purchased from Sigma-Aldrich, St Louis, MO, USA.
Spectrophotometric Assay of Drug
Stock solution of acetazolamide (1000 μg/ml) was prepared in methanol. Serial concentrations ranging from 4 to 20 µg/ml in methanol or from 2 to 14 µg/ml in simulated tear fluid were prepared from this methanolic stock solution. The prepared diluted solutions were analyzed by UV spectrophotometry at λ max of 268 nm using UV-spectrophotometer (Thermo, Evo300pc, USA). The absorbance of each concentration was recorded and plotted to construct the calibration curve.
Solubility Determination in Different Oils And surfactants
The solubility of acetazolamide in castor oil, olive oil, sesame oil, sunflower oil, Tween 20, and Tween 80 was determined. This was done by adding excess acetazolamide to the selected oils/surfactant. The prepared oil/drug mixture and surfactant/drug mixtures were stirred at 25 ºC for 48 h using magnetic stirrer (MSH-20A). After equilibrium, the content of each vial was centrifuged for 5 min. For further clarification, the separated supernatant was filtered using a membrane filter (0.45 µm). Sample from the filtered supernatant was diluted with methanol before acetazolamide quantification by UV spectrophotometer.
Construction of Pseudo Ternary Phase Diagram
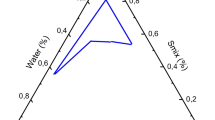
Pseudo ternary phase diagrams were constructed at ambient temperature using the well-established water titration method [28,29,30,31,32]. They were constructed utilizing either castor oil or olive oil as the oily phase, Tween 80 as the surfactant, and sodium carbonate solution as aqueous phase. Sodium carbonate solution (5.3% w/v) was prepared by dissolving 5.3 g sodium carbonate in 100 ml distilled water. This solution was selected as aqueous phase for better drug solubilization as the solubility of acetazolamide is pH dependent with higher solubility in alkaline medium. This will ensure incorporation of acetazolamide in the internal phase of w/o microemulsion. The selected oil and surfactant were mixed at weight ratios of 0.25:4.75, 0.5:4.5, 1:4, 1.5:3.5, 2:3, 2.5:2.5, 3:2, 3.5:1.5, 4:1, 4.5:0.5, and 4.75:0.25. Each mixture was titrated with the prepared sodium carbonate solution and the obtained system was characterized visually after equilibration. Phase changes upon increasing aqueous content was observed. The developed systems were characterized as ME if they were transparent one phase fluid systems. Viscous and opaque system showing oil streaks was considered either liquid crystal (LC) or gel phase. The former was identified based on its fluidity with the latter being selected based on its resistance to flow when tilted to an angle of 45°. Milky or turbid dispersion being mentioned as coarse emulsion [32].
Preparation of Ocular Formulations
Ocular formulations were selected from the constructed pseudo ternary phase diagram and the composition of these formulations is presented in Table I. This selection considered the existence of formulation close to the border between the ME and LC zone to allow phase transition after mixing with small amount of tear fluid. The formulations containing the drug at a concentration of 1% w/v were prepared by dissolving the drug in the required amount of sodium carbonate solution. Then, the required amount of surfactant was added to the prepared drug dispersion with mixing. After that, the oil was added, and mixing was continued till complete dissolution of the drug. The control employed aqueous drug solution (1% w/v) which was dissolved in sodium carbonate solution. Drug concentration was selected based on published article on topical ocular delivery of acetazolamide [2]. All formulations were freshly prepared to avoid stability issues (if any).
Characterization of the Prepared Formulations
PH Measurement
Measurements of pH values of the prepared microemulsion formulations were conducted using pH meter (HANNA pH 211 Microprocessor pH Meter).
Physical Stability Study
The physical stability of the prepared microemulsion formulations was evaluated using centrifugation method. The formulations were centrifuged by bench top centrifuge (Model 80–2, Taiwan) at speed of 4000 rpm at 25 ºC. After 30 min of centrifugation, the formulations were visually examined for any alteration in their homogeneity. The same method was employed in other research work [25].
Conductimetric Characterization of the Prepared Formulations
To determine the location of the aqueous phase within the prepared systems, the electrical conductivity of the selected formulation was recorded by electrical microprocessor conductivity meter (Hanna-HI 2300).
Rheological Measurements
The viscosity and flow properties of the prepared formulations were determined using a RheoWin HAAKE Visco Tester IQ Thermo Fisher “Vane geometry and universal conminer holder” SN 15,063,200,722,241,020. The viscosity value at fixed shear rate was utilized for comparing different formulations.
Transmission Electron Microscopy
The morphology of the tested microemulsions was examined using transmission electron microscope (JEOL-JSM-1400 PLUS, Tokyo, Japan). The tested formulation was loaded on copper grid before 5 min staining with uranyl acetate followed by lead citrate for 2 min. The droplets of the investigated microemulsion were photographed after adjusting the magnification power according to the size of the droplets. The same approach was utilized in another research for photographing of ME [25].
Polarized Light Microscopy
The morphology of the liquid crystal formulations was observed using polarized light microscope (Biological microscope, Model Star E5, Germany). The tested formulation was spread as thin film on glass slide and was photographed after adjusting the magnification power.
Particle Size Analysis
The droplet size and polydispersity index (PDI) of the prepared microemulsion formulations was measured at 25°C using dynamic light scattering technique (DLS). The measurement employed a Nano-ZS Zetasizer (Malvern Instruments Ltd., Worcestershire, UK). The angle of scattering was 90° and the samples of microemulsion were not diluted to avoid phase transition during the analysis. The data were given as the average particle size ± the standard deviation (Z-average ± SD). The method was utilized in another research for droplet size determination of ME [5].
In vitro Drug Release Study
The in vitro release of acetazolamide from the prepared formulations was monitored using semipermeable dialysis bags (cellulose tubing, M.W. cutoff 14,000 Daltons, Sigma-Aldrich, St. Louis, MO, USA). This method was utilized previously in several research work [33,34,35]. The cellulose tube was soaked in distilled water for 24 h before being cut into sacs, each having 5 cm in length. Soaking the membrane in distilled water allows for complete swelling to provide constant pore diameter. Each dialysis bag was filled with 2.0 mL of aqueous drug solution, microemulsion or liquid crystalline formulation and was firmly tied. The loaded sac was then immersed in a beaker containing 200 mL of simulated lacrimal fluid previously heated to 37 ± 0.5°C. Lacrimal fluid comprised sodium chloride, potassium chloride, calcium chloride anhydrous, sodium bicarbonate, and water. The beakers were maintained in thermostated water bath kept at 37 ± 0.5°C with occasional mixing. Samples from the receptor fluid were withdrawn at time intervals of 0.5,1, 2, 3, 4, 5, 6, and 7 h. These samples were analyzed for acetazolamide content by UV spectrophotometric method. To maintain sink condition and ensure fixed volume, the withdrawn samples were replenished with fresh receptor fluid. For construction of the release profile, the percentage of acetazolamide released was plotted as a function of time. The release kinetics were investigated by fitting the release data to different kinetic models including zero-order, first-order, and Higuchi kinetic models.
In vivo Study
This study was conducted using 16 New Zealand white male albino rabbits weighting 2 ± 0.5 kg. The rabbits were handled according to the National Institute of Health guide (NIH) for the care and manipulation of laboratory animals. The ethical committee of Faculty of Pharmacy, Tanta University approved this research protocol under the approval number of 17,122,019. The intraocular pressure (IOP) of the tested rabbits was measured to ensure its normal value. Ocular hypertension was induced by intraocular administration of 0.1% dexamethasone eye drops 3 times daily for 1 week [2, 36]. The IOP of the rabbits was monitored using a Schiotz tonometer (Schiotz tonometer, Riester, Germany). The animals were injected with ketamine HCl solution (25 mg) before starting the measurement to allow easy handling of rabbits. The rabbits that recorded high IOP were divided into 4 groups each group comprised 4 rabbits. Group I was treated with single dose (2 drops) of olive oil-based microemulsion containing 1% w/w acetazolamide according to the composition in Table I. Group II was treated with single dose (2 drops) of castor oil-based microemulsion containing the same concentration of acetazolamide according to the composition in Table I. Group III was treated with 1% w/v acetazolamide solution in 5.3% w/v sodium carbonate solution and served as positive control. The same volume was applied from the test and control to allow direct comparison. Group IV was kept as negative control group which did not receive any treatment. IOP was monitored at 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 h after drug administration. Each time point was average of 3 measurements conducted at the same time. The recorded IOP was plotted as function of time and this plot was utilized to compute the area above the curve (AAC 0–10) by non-linear trapezoidal. This parameter was employed to compare the efficacy of different formulations.
The percentage decrease in IOP for each time point was calculated as follow:
This equation was reported in literatures [2]. The calculated % decrease in IOP was plotted versus time to construct the therapeutic profile. This therapeutic profile was utilized to compute the pharmacodynamic parameters including area under the curve (AUC 0–10) which was computed by non-linear trapezoidal strategy, and the time required to achieve maximum reduction in IOP (Tmax). Maximum % decrease in IOP which represents the greatest reduction in IOP was also utilized for comparing the efficiency of different formulations.
Ocular Irritation Studies
The ocular irritation study employed 8 rabbits divided into 4 groups which were treated according to standard laboratory guidelines according to a protocol approved by ethical committee of faculty of pharmacy, Tanta university (approval number: 17122019). The non-medicated microemulsion formulation or its corresponding oil (2drops) was inserted in the right eye of the rabbits with the left eye serving as control. The eye was visually observed for redness which was taken as a sign for ocular irritation for 24 h. Moreover, phenol red thread test was employed to monitor the rate of tear flow.
The phenol red thread test employed a phenol red impregnated cotton thread which was inserted into the inferior fornix of the conjunctival sac. This thread is pH sensitive that alters its color from yellow to red color when wetting by tears. After 15 s, the red portion of the thread was recorded. A length of the wet red thread of less than 10 mm in 15 s indicates tear deficiency [37]. The base line tear volume was established by inserting the thread into the inferior fornix of the conjunctival sac of the left eye of the rabbit and recording the colored length 15 s after insertion [37, 38]. The formulation under assessment was applied into the lower fornix of the conjunctival sac of the same eye. After 5 min post instillation, phenol red thread test was conducted to evaluate the change in tear volume and the difference in the measured wetted length after and before instillation was computed. The greater the difference the more irritant will be the instilled system.
Statistical Analysis
Statistical analysis of the in vitro release data and in vivo data was performed using Kruskal Wallis test with Tukey’s multiple comparison was employed as post hoc test to explore the significance between the formulations.
Results and Discussion
Calibration Curve and Method Validation of Acetazolamide
The calibration curve was constructed by plotting the measured drug absorbance as a function of acetazolamide concentration. The constructed calibration curves in methanol or simulated tear fluid are shown in Fig. 1. This calibration curves were linear within the tested concentration range. The linearity was proven from the recorded value of the correlation coefficient which was computed after fitting the data to straight line equation. The calibration curve equation in methanol was Y = 0.0368x + 0.0591 and the equation of the calibration curve constructed in simulated tear fluid was Y = 0.0551x + 0.0233.
Solubility Studies
Solubility studies of drug in various oils and surfactant were conducted and the results are presented in Table II. Olive oil and castor oil showed higher drug solubilization power compared to other vegetable oils. Tween 80 showed the highest solubility of acetazolamide. The recorded solubility values correlate with the published data on the same drugs using the same vehicles [39]. Accordingly, olive oil and castor oil were selected as oily phase and Tween 80 was selected as surfactant for construction of pseudo ternary phase diagrams.
Pseudo-ternary Phase Diagram
The phase diagrams of olive oil or castor oil with the selected surfactant (Tween 80) were constructed as shown in Fig. 2. These phase diagrams were used to investigate the phase behavior of the developed systems after being mixed with increasing concentration of water. The composition of the formulations for ocular drug application were selected based on the constructed phase diagram. In addition, the phase diagrams were utilized to predict the phase transition of the formulations upon mixing with tear fluid after ocular use. Regarding olive oil-based phase diagram, four discrete zones were shown. These phases included microemulsion zone, liquid crystalline system that developed upon dilution of ME with water, stable gel phase after further dilution with water and finally coarse emulsion zone upon additional dilution. The ME zone occupied 13% of the total area of the constructed phase diagram while the LC zone occupying 25.5%. The gel zone occupied about 11% and the EM zone covered 50.5% of the constructed diagram. Phase diagrams-based on olive oil were reported in several research works [37, 40]. However, these previous investigations did not utilize sodium carbonate solution as aqueous phase which hampered direct comparison between the phase diagrams.
Like olive oil, castor oil phase diagram also showed four zones which included ME zone (occupied 10% of the total area of the phase diagram), the LC zone (occupied 23.5%), the gel zone (occupied 8.5%), and the EM zone (occupied 58%). Castor oil was utilized as the oily phase with other investigators in formulation of microemulsion, but the authors employed Tween 80 as surfactant in presence of ethanol as cosurfactant [41].
Characterization of the Selected Formulations
The pH values of the tested ME formulations depended on the nature of aqueous phase. Using water as the aqueous phase of ME provided formulations with pH of 5.5 and 6.3 for olive oil and castor oil-based ME, respectively. These pH values were modulated after replacing water with sodium carbonate solution to record 6.6 and 7.2 for olive oil and castor oil-based ME, respectively. These values lie within the physiological range of the eye (6.5 to 7.6); hence, it would not cause any irritation upon administration [42,43,44].
Physical stability testing indicated no phase separation in the tested microemulsion formulations after being centrifuged for 30 min. Sample homogeneity without phase separation after centrifugation confirmed physical stability of the prepared olive oil or castor oil-based microemulsions.
The electrical conductivity values of the tested ME formulations were measured to be 6.4 and 2.1 µs for olive oil and castor oil-based systems, respectively. These recorded low electrical conductivity values indicated that the ME systems were in the form of W/O microemulsion. This is because, the electrical conductivity value depends on the water content of the tested formulations (the lower the water content the lower the electrical conductivity values). Water was added to ME formulations as indicated by the line plotted on the phase diagrams (Fig. 2). Increasing water content developed LC systems which showed an increase in the electrical conductivity to reach 30 and 18 µ s for olive oil and castor oil-based LC, respectively. This is acceptable taking into consideration the nature of LC system which is a transition state between W/O ME and O/W coarse emulsion. Additional increase in water concentration till conversion to coarse emulsion (EM) resulted in significant increase in the electrical conductivity. The electrical conductivity was recorded to be 510 µs and 450 µs for olive oil and castor oil coarse emulsions, respectively. These results confirm the type of the tested microemulsion formulations as W/O and correlate with the phase transition behavior which eventually transform into an O/W coarse emulsion. These collected data indicated different structural arrangement of the employed ternary system upon increasing water content. The system converted from W/O system to lamellar liquid crystalline system prior to phase inversion into an O/W system. This behavior was previously reported with other phase transition systems [37, 41,42,43,44,45].
The viscosity and rheological behavior of the tested formulations have great impact on drug release, onset, and duration of action. Accordingly, the viscosity and rheological behavior of the tested ME and LC systems were determined, and the results are shown in Table III and Fig. 3. The tested ME formulations exhibited Newtonian flow pattern with the viscosity values measured to be 678.5 and 737.5 centipoise for castor and olive oil based ME respectively. The LC systems showed non-Newtonian, pseudoplastic flow behavior in which the viscosity values decreased upon increasing the shear rate (shear thinning behavior). This behavior is advantageous with ocular dosage form which allows natural movement of the eye lid. Similar rheological behavior was reported by other researchers for ME and LC systems [24, 37]. For comparison, the viscosity of the tested ME and LC formulations was recorded at fixed shear rate value 50 1/s and the results are presented in Table III. The viscosity of LC formulations was significantly higher than the viscosity of ME formulations. These results are expected based on the semisolid crystalline structure of LC system compared with ME system. Several literatures reported similar behavior for ME and LC prepared using various oil/surfactant systems [37, 46,47,48]. The recorded rheological properties of LC indicated tolerability of the selected ME system which is expected to form LC with a shear thinning behavior after mixing with tears.
Figure 4a and b show the transmission electron micrographs of castor oil-based microemulsion and olive oil-based microemulsion. These micrographs revealed the spherical nature of the tested microemulsions with the droplets size being in nano range. Similar TEM micrographs were taken previously for microemulsion systems [49]
Figure 4c and d show the photographs of castor oil-based and olive oil-based liquid crystals. These photographs revealed the existence of oil strokes which are characteristic for liquid crystals
Particle size analysis showed that the average droplet size values were 79.5 nm (SD = 4.8) and 96.5 nm (SD = 7.5) for castor oil-based microemulsion and olive oil-based microemulsion, respectively (Fig. 4e, f). The figures show narrow particle size distribution. This distribution and the recorded size values correlate with published figures of microemulsions [27].
In vitro Drug Release
The in vitro release profiles of acetazolamide from ME, LC and aqueous drug solution are shown in Fig. 5. The collected release data were used to determine the drug release kinetics by fitting to different kinetic models with the calculated correlation coefficient values being presented in Table III. The model recorded the highest value of correlation coefficient (best fit) was taken as the kinetic model for drug release. The best fit model was utilized to calculate acetazolamide release rate. With respect to acetazolamide release from castor oil or olive oil-based ME and LC formulations, the regression analysis of the collected data showed best fit to the Higuchi diffusion model (Table III and Fig. 5). This release behavior suggests the existence of matrix structure which is obvious in case of LC system which is gel like structure. For the liquid ME the release behavior can be attributed to the nature of the aqueous phase employed in developing the tested ME or LC systems which comprises sodium carbonate solution for enhancing drug solubility overcoming its hydrophobic nature. This allowed the localization of the drug in the internal aqueous phase of the microemulsion resulting in diffusion-controlled release kinetics. Similar release pattern was stated for ME and LC formulations of pilocarpine hydrochloride and timolol maleate which were similarly included in the internal aqueous phase of the ME [24, 33]. As expected, the aqueous solution showed zero order release kinetics of acetazolamide confirming drug release at constant rate. This behavior was reported previously with other drugs from their aqueous solutions [37, 50].
Regarding acetazolamide release rate, it was evident that the release rate from ME and LC formulations was significantly slower compared with the aqueous drug solution (p < 0.01). The release rate from LC formulations was recorded to be the slowest (p < 0.05) (Fig. 5). This release pattern can be explained based on the recorded viscosity of the tested formulation. The liquid crystalline formulations that recorded the highest viscosity values produced the slowest release rate followed by the microemulsion system then the aqueous drug solution (Table III and Fig. 5). The release rate from castor oil-based systems either ME or LC was higher than that of olive oil-based systems. This correlates with the recorded viscosity values for both systems.
In vivo Evaluation
The IOP reducing efficiency after ophthalmic application of acetazolamide in olive oil ME, castor oil ME and drug solution (control) was recorded. This employed male albino rabbits with ocular hypertension being induced using dexamethasone eye drops. The IOP was plotted as function of time and these plots are shown in Fig. 6. In addition, these plots were used to calculate the pharmacodynamic parameters which were utilized for comparing the effectiveness of the tested formulations (Table IV and Fig. 7). Ocular application of acetazolamide formulated in either olive oil or castor oil based MEs or acetazolamide aqueous solution resulted in significant reduction in IOP from baseline compared with negative control group (Fig. 7). The time showing maximum decrease in IOP (Tmax) value for either olive oil or castor oil-based ME was 3 h while that for acetazolamide aqueous solution was 1 h (Table IV). This indicates faster onset of action for drug solution compared with ME formulations. This can be explained based on the recorded higher release rate of acetazolamide from its aqueous solution compared with ME systems. Figure 7 revealed sustained drug effect for ME formulations compared with aqueous drug solution with olive oil-based system being superior. The duration of action was prolonged and the reduction in IOP continued for up to 10 h in case of ME formulations, while that of aqueous drug solution continued only for 4–5 h. For comparing the efficiency of different formulations, the area above curve parameter (AAC0–10) for IOP response from the baseline and the area under the curve AUC0–10 from % decrease in IOP versus time plot were calculated (Table IV). The results demonstrated twofold and threefold increase in AAC0–10 and AUC0–10 in case of castor oil ME and olive oil ME compared with aqueous acetazolamide solution, respectively (p < 0.05) (Table IV). Ocular application of olive oil ME showed a trend of higher AUC0–10 value compared with castor oil-based ME (p > 0.05). The enhanced efficacy of acetazolamide from ME formulations can be accredited to possible penetration enhancing effect of the ME components. The superiority of olive oil-based ME can be explained based on its better penetration enhancing properties owing to the presence of oleic acid as the main component. Similar explanation was reported with other researchers [37]. In addition to the oil, the presence of surfactant in ME formulations provides a mean for enhancing drug corneal permeation by disrupting tight junctional complexes [51]. Moreover, the amphiphilic nature of ME allowed for easy mixing with the pre-corneal tear film which has lipophilic exterior and aqueous interior with subsequent greater chance for permeation through the cornea [24]. The prolonged reduction in IOP after ocular application of ME can be attributed to phase transition to the more viscous liquid crystalline form upon mixing with tear fluid. This phase transition is expected to take place easily based on composition of the tested ME which is located on the phase diagram close to border between the ME and LC zones (Fig. 2). Transformation to viscous LC can prolong ocular contact time resulted in sustained duration of action. This pattern is highly advantageous to maintain constant low IOP levels in glaucoma patients. Similar findings were reported for W/O ME system [2, 24, 33, 52].
Ocular Irritation Studies
Enhancing the pharmacological response of a drug while maintaining ocular safety are the main criteria for successful development of ocular delivery system. Consequently, the microemulsion formulations and their oil contents were evaluated for their impact on ocular irritation by visual monitoring of redness and estimation of tear flow rate. The later was monitored using phenol red thread test and the difference in the wetted portion length of the thread before and after instillation of the formulations was computed and used for comparison. Visual inspection of rabbit eye after being instilled with the tested ME showed slight redness compared with the control eye. This redness reduced gradually, and the eye showed complete recovery after 24 h in case of castor oil based microemulsion with slight redness in case of olive oil based microemulsion. The individual oils either olive or castor oil were more irritating compared with their corresponding ME formulations. They showed more eye redness and slower recovery to the normal state. Irritation of pure oils has been recorded previously by other investigators [53].
Regarding the tear volume measurements, the average differences in the wetted length of the phenol red threads were 11.9 ± 1.6, 12.4 ± 0.9, 4.9 ± 0.8, 6.4 ± 1.4, and 8.9 ± 1.1 mm for pure castor oil, pure olive oil, castor oil ME, olive oil ME, and drug solution. These results reflected slower tear flow rate from ME formulations compared with pure oils or even aqueous acetazolamide solution. This reflects the ability of ME to improve the safety profiles of its components. Similar results were informed previously [25, 37, 54]. The tear flow rate was recorded to be high in case of olive oil compared with castor oil formulated in the form of ME. This indicates better ocular safety for castor oil-based systems. The same findings were recorded previously by El Agamy and El Maghraby [37].
Conclusion
Microemulsion formulations were developed using either olive oil or castor oil as oily phase. The developed microemulsion revealed slower acetazolamide release rate compared with aqueous drug solution. The developed liquid crystal formulations showed slow-release rate which was attributed to the recorded high viscosity value. Ocular application of acetazolamide either in aqueous solution or in the form of microemulsion resulted in significant reduction in the intraocular pressure. The aqueous drug solution produced faster onset of action owing to faster drug release. The application of microemulsion formulations enhanced the efficacy and prolonged the drug duration of action with the olive oil-based ME system being superior.
The constructed pseudo-ternary phase diagrams using a olive oil (oily phase), Tween 80 (surfactant) and sodium carbonate solution (aqueous phase) b castor oil (oily phase), Tween 80 (surfactant) and sodium carbonate solution (aqueous phase). The star represents the point corresponding to the composition of the selected microemulsion formulations and the line indicate the phase change upon dilution with water
Transmission electron micrographs of a castor oil-based microemulsion and b olive oil-based microemulsion; polarized light photomicrographs for c castor oil-based liquid crystals and d olive oil-based liquid crystals; and size distribution chart for e castor oil-based microemulsion and f olive oil-based microemulsion
References
Loiselle AR, de Kleine E, van Dijk P, Jansonius NM. Intraocular and intracranial pressure in glaucoma patients taking acetazolamide. PLoS One. 2020;15. https://doi.org/10.1371/journal.pone.0234690.
Morsi N, Mohamed MI, Refai H, El Sorogy HM. Nanoemulsion-based electrolyte triggered in situ gel for ocular delivery of acetazolamide. Eur J Pharm Sci. 2017;104:302–14. https://doi.org/10.1016/j.ejps.2017.04.013.
Hathout RM, Mansour S, Mortada ND, Guinedi AS. Liposomes as an ocular delivery system for acetazolamide: in vitro and in vivo studies. AAPS Pharm Sci Tech. 2007;8:1. https://doi.org/10.1208/pt0801001.
Gambhire S, Bhalerao K, Singh S. In situ hydrogel: different approaches to ocular drug delivery. Int J Pharm Sci. 2013;5:27–36.
Morsi NM, Mohamed MI, Refai H, El Sorogy HM. Nanoemulsion as a novel ophthalmic delivery system for acetazolamide. Int J Pharm Sci. 2014;6:227–36.
Kakkar S, Kaur I. Spanlastics-a novel nanovesicular carrier system for ocular delivery. Int J Pharm. 2011;413:202–10. https://doi.org/10.1016/j.ijpharm.2011.04.027.
El Maghraby GM, Arafa MF, Essa EA. Phase transition microemulsions as drug delivery systems. In: Inamuddin, Asiri AM, Mohammad A, editors. Applications of nanocomposite materials in drug delivery. Woodhead Publ. 2018. p. 787–803.
Vyas SP, Mysore N, Jaidey V, Venhastesan N. Discoidal niosomes based controlled ocular delivery of timolol maleate. Pharmazie. 1998;53:466–9.
Pignatello R, Bucolo C, Ferrara P, Maltese A, Puglisi G. Eudragit RS100® nanosuspensions for the ophthalmic controlled delivery of ibuprofen. Eur J Pharm Sci. 2002;16:53–61. https://doi.org/10.1016/s0928-0987(02)00057-x.
Pepic I, Jalsenjak N, Jalsenjak I. Micellar solutions of triblock copolymer surfactants with pilocarpine. Int J Pharm. 2004;272:57–64. https://doi.org/10.1016/j.ijpharm.2003.11.032.
Aggarwal D, Kaur IP. Improved pharmacodynamics of timolol maleate from a mucoadhesive niosomal ophthalmic drug delivery system. Int J Pharm. 2005;290:155–9. https://doi.org/10.1016/j.ijpharm.2004.10.026.
Ali J, Fazil M Qumbar M, Khan N, Ali A. Colloidal drug delivery system: amplify the ocular delivery. Drug Deliv. 2016;23:710–26. https://doi.org/10.3109/10717544.2014.923065.
Durrani AM, Farr SJ, Kellaway IW. Influence of molecular weight and formulation pH on the precorneal clearance rate of hyaluronic acid in rabbit eye. Int J Pharm. 1995;118:243–50.
Khalil IA, Saleh B, Ibrahim DM, Jumelle C, Yung A, Dana R, et al. Ciprofloxacin-loaded bioadhesive hydrogels for ocular applications. Biomater Sci. 2020;8:5196–209. https://doi.org/10.1039/D0BM00935K.
Miller SC, Donovan M. Effect of poloxamer 407 gel on the miotic activity of pilocarpine nitrate in rabbits. Int J Pharm. 1982;12:147–52. https://doi.org/10.1016/0378-5173(82)90114-4.
Majeed A, Khan N. Ocular in situ gel: an overview. J drug Deliv Ther. 2019;9:337–47. https://doi.org/10.22270/jddt.v9i1.2231.
Cassano R, Gioia ML, Trombino S. Gel-based materials for ophthalmic drug delivery. Gels. 2021;7:130. https://doi.org/10.3390/gels7030130.
Wafa HG, Essa EA, El-Sisi AE, El Maghraby GM. Ocular films versus film forming liquid systems for enhanced ocular drug delivery. Drug Deliv Transl Res. 2021;11:1084–95. https://doi.org/10.1007/s13346-020-00825-1.
Gurny R, Boye T, Ibrahim H. Ocular therapy with nanoparticulate systems for controlled drug delivery. J Control Release. 1985;2:353–61. https://doi.org/10.1016/0168-3659(85)90057-4.
Unterman SR, Rootman DS, Hill JM, Parelman JJ, Thompsom HW, Kaufman HE. Collagen shield drug delivery: therapeutic concentrations of tobramycin in the rabbit cornea and aqueous humor. J Cataract Refract Surg. 1988;14:500–4. https://doi.org/10.1016/s0886-3350(88)80006-3.
Kaufman HE, Stuinemann TL, Lehman E, Thompson HW, Varnell ED, Jacob-Labarre JT, et al. Collagen-based drug delivery and artificial tears. J Ocul Pharmacol. 1994;10:17–27. https://doi.org/10.1089/jop.1994.10.17.
Vandamme TF. Microemulsions as ocular drug delivery systems: recent developments and future challenges. Prog Retin Eye Res. 2002;21:15–34. https://doi.org/10.1016/s1350-9462(01)00017-9.
Alany RG, Rades T, Nicoll J, Tucker IG, Davies NM. W/O microemulsions for ocular delivery: evaluation of ocular irritation and precorneal retention. J Control Release. 2006;111:145–52. https://doi.org/10.1016/j.jconrel.2005.11.020.
Chan J, Maghraby GM, Craig JP, Alany RG. Phase transition water-in-oil microemulsions as ocular drug delivery systems: in vitro and in vivo evaluation. Int J Pharm. 2007;328:65–71. https://doi.org/10.1016/j.ijpharm.2006.10.004.
Habib F, El-Mahdy M, Maher S. Microemulions for ocular delivery: evaluation and characterization. J Drug Deliv Sci Tech. 2011;21:458–89. https://doi.org/10.1016/S1773-2247(11)50078-4.
Gautam N, Kesavan K. Development of microemulsions for ocular delivery. Ther deliv. 2017;8:313–30. https://doi.org/10.4155/tde-2016-0076.
El Maghraby GM. Transdermal delivery of hydrocortisone from eucalyptus oil microemulsion: effects of cosurfactants. Int J Pharm. 2008;355:285–92. https://doi.org/10.1016/j.ijpharm.2007.12.022.
El Maghraby GM, Arafa MF, Osman MA. Microemulsion for simultaneous transdermal delivery of benzocaine and indomethacin: in vitro and in vivo evaluation. Drug Dev Ind Pharm. 2014;40:1637–44. https://doi.org/10.3109/03639045.2013.841186.
Gan L, Gan Y, Zhu C, Zhang X, Zhu J. Novel microemulsion in situ electrolyte-triggered gelling system for ophthalmic delivery of lipophilic cyclosporine A: in vitro and in vivo results. Int J Pharm. 2009;365:143–9. https://doi.org/10.1016/j.ijpharm.2008.08.004.
Fathalla D, Soliman GM, Fouad EA. Development and in vitro/in vivo evaluation of liposomal gels for the sustained ocular delivery of latanoprost. J Clin Exp Ophthalmol. 2015;6:390. https://doi.org/10.4172/2155-9570.1000390.
Tiwari R, Pandy V, Asati S, Soni V, Jain D. Theraputic challenges in ocular delivery of lipid based emulsion. Egypt J Basic Appl Sci. 2018;5:121–9. https://doi.org/10.1016/j.ejbas.2018.04.001.
El Maghraby GM. Self-microemulsifying and microemulsion systems for transdermal delivery of indomethacin: effect of phase transition. Colloids Surf B. 2010;75:595–600. https://doi.org/10.1016/j.colsurfb.2009.10.003.
Hegde RR, Bhattacharya SS, Verma A, Ghosh A. Physicochemical and pharmacological investigation of water/oil microemulsion of non-selective beta blocker for treatment of glaucoma. Curr Eye Res. 2014;39:155–63. https://doi.org/10.3109/02713683.2013.833630.
Subongkot T, Ngawhirunpat T. Development of a novel microemulsion for oral absorption enhancement of all-trans retinoic acid. Int J Nanomed. 2017;12:5585–99. https://doi.org/10.2147/IJN.S142503.
Zheng Y, Xu G, Ni Q, Wang Y, Gao Q, Zhang Y. microemulsion delivery system improves cellular uptake of genipin and its protective effect against Aβ1-42-induced PC12 cell cytotoxicity. Pharmaceutics. 2022;14:617. https://doi.org/10.3390/pharmaceutics14030617.
Whitlock NA, Mcknight B, Corcoran KN, Rodriguez LA, Rice DS. Increased intraocular pressure in mice treated with Dexamethsone. Invest Ophthalmol Vis Sci. 2010;51:6496–503. https://doi.org/10.1167/iovs.10-5430.
El Agamy HI, El Maghraby GM. Natural and synthetic oil phase transition microemulsions for ocular delivery of tropicamide: efficacy and safety. J App Pharm Sci. 2015;5:067–75. https://doi.org/10.7324/JAPS.2015.58.S11.
Chan J, El Maghraby GM, Craig JP, Alany RG. Effect of water-in-oil microemulsions and lamellar liquid crystalline systems on the precorneal tear film of albino New Zealand rabbits. Clin Ophthalmol (Auckland, NZ). 2008;2(1):129. https://doi.org/10.2147/OPTH.S2032.
Tarkeshwar PS, Sharma AK. Formulaion, development and In-vitro evaluation of acetazolamide self micro emulsifying drug delivery system (SMEDDS) to improve the solubility and bioavailability. World j pharm pharm sci. 2017;6:1000–27. https://doi.org/10.1038/s41598-020-79940-5.
Sha X, Wu J, Chen Y, Fang X. Self Microemulsifying drug delivery system for improved oral Bioavailability of Probucol: preparation and evaluation. Int J Nanomed. 2012;7:705–12. https://doi.org/10.2147/IJN.S28052.
Nazar MF, Khan AM, Shah SS. Microemulsion system with improved loading of piroxicam: a study of microstructure. AAPS PharmSciTech. 2009;10:1286–94. https://doi.org/10.1208/S12249-009-9328-9.
Ableson MB, Udell IJ, Weston JH. Normal human tear Ph by direct measurement. Arch Ophthalmol. 1981;99:301. https://doi.org/10.1001/archopht.1981.03930010303017.
Ammar HO, Salama HA, Ghorab M, Mahmoud AA. Nanoemulsion as a potential ophthalmic delivery system for dorzolamide hydrochloride. AAPS PharmSciTech. 2009;10:808–19. https://doi.org/10.1208/s12249-009-9268-4.
Preetha J, Karthika K, Rekha N, Elshafie K. Formulation and evaluation of in situ ophthalmic gels of Diclofenac sodium. J Chem Pharm Res. 2010;2:528–35.
Alany RG, Tucker IG, Davies NM, Rades T. Characterizing colloidal structures of pseudoternary phase diagrams formed by oil/water/amphiphile systems. Drug Dev Ind Pharm. 2001;27:31–8. https://doi.org/10.1081/ddc-100000125.
El Maghraby GM, Bosela AA. Investigation of self microemulsifying and microemulsion systems for protection of prednisolone from gamma radiation. Pharm Dev Technol. 2011;16:237–42. https://doi.org/10.3109/10837451003610829.
El Maghraby GM. Occlusive and non-occlusive application of microemulsion for transdermal delivery of progesterone: mechanistic studies. Sci Pharm. 2012;80:765–78. https://doi.org/10.3797/scipharm.1201-01.
Ren X, Svirskis D, Alany GR, Shoshtari SZ, Wu Z. In-situ phase transition from microemulsion to liquid crystal with the potential of prolonged parenteral drug delivery. Int J Pharm. 2012;431:130–7. https://doi.org/10.1016/j.ijpharm.2012.04.020.
Malakar J, Basu A, Kumar NA. Candesartan cilexetil microemulsions for transdermal delivery: formulation, in-vitro skin permeation and stability assessment. Curr Drug Deliv. 2014;11(3):313–21. https://doi.org/10.2174/1567201810666131211110517.
Eid RK, Arafa MF, Ashour DS, Essa EA, Zoghroban HS, Issa YA, et al. Eudragit coated microemulsion for enhanced efficacy of spiramycin against toxoplasmic encephalitis. J Drug Deliv Sci Technol. 2022;69. https://doi.org/10.1016/j.jddst.2022.103137
Kaur IP, Smitha R. Penetration enhancers and ocular bioadhesives: two new avenues for ophthalmic drug delivery. Drug Dev Ind Pharm. 2002;28:353–69. https://doi.org/10.1081/ddc-120002997.
Teba HE, Kalil IA, El Sorogy HM. Novel cubosome based system for ocular delivery of acetazolamide. Drug Deliv. 2021;28:2177–86. https://doi.org/10.1080/10717544.2021.
Burnett CL, Heldreth B, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler DC, Marks Jr JG, Shank RC, Slaga TJ, Snyder PW. Safety assessment of pegylated oils as used in cosmetics. Int J Toxicol. 2014;33(4_suppl):13S-39S. https://doi.org/10.1177/1091581814546337.
Hegde RR, Verma A, Ghosh A. Microemulsion: new insights into the ocular drug delivery. Int Sch Res Notices. 2013;2013. https://doi.org/10.1155/2013/826798. Print 2013.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Lubna M. Abd El Wahab: investigation, data curation, visualization and writing original draft.
Ebtessam A. Essa: conceptualization, methodology, visualization, writing, reviewing, and editing.
Gamal M. El Maghraby: conceptualization, methodology, visualization, supervision, writing, reviewing, and editing.
Mona F. Arafa: methodology, data curation, visualization, writing, reviewing, and editing.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The study employed rabbits which were treated according to standard laboratory guidelines according to a protocol approved by ethical committee of faculty of pharmacy, Tanta university (approval number: 17122019).
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abd El Wahab, L.M., Essa, E.A., El Maghraby, G.M. et al. The Development and Evaluation of Phase Transition Microemulsion for Ocular Delivery of Acetazolamide for Glaucoma Treatment. AAPS PharmSciTech 24, 1 (2023). https://doi.org/10.1208/s12249-022-02459-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02459-7