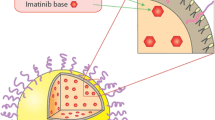
Abstract
An intraarticular, liposphere-based, formulation of Imatinib mesylate for weekly administration was developed. Lipospheres were prepared using double emulsion technique using dierucoyl phosphatidylcholine, 1,2-dipalmitoyl-sn-glycero-3-phospho-rac-(1-glycerol) sodium salt), cholesterol, and tricaprylin as lipid phase in dichloromethane in a four-step process. Primary emulsion, formed using a high-pressure homogenizer, was diluted using a secondary aqueous phase in an Inline mixer to form the liposomal dispersion. Nitrogen flushing was done to remove dichloromethane, and the dispersion was finally centrifuged and adjusted for potency. The amount of cholesterol and triglyceride was taken as formulation variables, and speed of homogenization was used as a process variable in the Box-Behnken design while particle size, % drug entrapment, and drug release at the end of 4 h and 5 days were taken as response variables. Multivariate data analysis grouped the variables in two latent variable sets, one based on the speed and the other on the composition of lipospheres. Multiple linear regression analysis was used to generate mathematical model for each response. Constraints were put on the values of responses, as per the requirements of the final product, and the “freedom to operate” design space was located using an overlay plot. The center point batch sufficed all the set criteria, and Monte Carlo simulations on the factor variables indicated a defect rate of 5%. The center point batch was characterized for viscosity, osmolality, pH, drug release, and lipocrit value. The dispersion was charged in a prefilled syringe and studied for stability. The product was found to be stable at 2–8°C over a period of 6 months.
Graphical abstract








Similar content being viewed by others
Abbreviations
- RA:
-
Rheumatoid arthritis
- IMB:
-
Imatinib mesylate
- OFAT:
-
One factor at a time
- DEPC :
-
Dierucoyl phosphatidylcholine
- DPPG:
-
1,2-Dipalmitoyl-sn-glycero-3-phospho-rac-(1-glycerol) sodium salt)
- HPLC:
-
High-performance liquid chromatography
- FTIR:
-
Fourier transform infrared
- DSC:
-
Differential scanning calorimetry
- BBD:
-
Box-Behnken design
- IMB-LPS:
-
IMB liposphere dispersion
- PPV:
-
Packed particle volume
- GC:
-
Gas chromatography
- PFS:
-
Prefilled syringe
- SEM:
-
Scanning electron microscopy
- PCA:
-
Principal component analysis
- EFA:
-
Exploratory factor analysis
- PS:
-
Particle size
- ZP:
-
Zeta potential
- EE:
-
Percentage entrapment efficiency
- DR4h:
-
Drug release at the end of 4 h
- DR5d:
-
Drug release at the end of 5 days
- CQA:
-
Critical quality attribute
- CMA:
-
Critical material attribute
- CPP:
-
Critical process parameter
- FTO:
-
Freedom to operate
- VIF:
-
Variance inflation factor
- LoF:
-
Lack of fit
- DR:
-
Defect rate
References
Cross M, Smith E, Hoy D, Carmona L, Wolfe F, Vos T, et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1316–22. https://doi.org/10.1136/annrheumdis-2013-204627.
Crowson CS, Matteson EL, Myasoedova E, Michet CJ, Ernste FC, Warrington KJ, et al. The lifetime risk of adult-onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis Rheum. 2011;63(3):633–9. https://doi.org/10.1002/art.30155.
Rheumatoid Arthritis In: Arthritis Information. https://www.hopkinsarthritis.org/arthritis-info/rheumatoid-arthritis/. Accessed 22 Aug 2022.
Bas DB, Su J, Wigerblad G, Svensson CI. Pain in rheumatoid arthritis: models and mechanisms. Pain Manag. 2016;6(3):265–84. https://doi.org/10.2217/pmt.16.4.
Eklund KK, Joensuu H. Treatment of rheumatoid arthritis with imatinib mesylate: clinical improvement in three refractory cases. Ann Med. 2003;35(5):362–7. https://doi.org/10.1080/07853890310001339.
Paniagua RT, Sharpe O, Ho PP, Chan SM, Chang A, Higgins JP, et al. Selective tyrosine kinase inhibition by imatinib mesylate for the treatment of autoimmune arthritis. J Clin Invest. 2006;116(10):2633–42. https://doi.org/10.1172/JCI28546.
Ando W, Hashimoto J, Nampei A, Tsuboi H, Tateishi K, Ono T, et al. Imatinib mesylate inhibits osteoclastogenesis and joint destruction in rats with collagen-induced arthritis (CIA). J Bone Miner Metab. 2006;24(4):274–82. https://doi.org/10.1007/s00774-006-0684-1.
Rai MF, Pham CT. Intra-articular drug delivery systems for joint diseases. Curr Opin Pharmacol. 2018;40:67–73. https://doi.org/10.1016/j.coph.2018.03.013.
Bowman S, Awad ME, Hamrick MW, Hunter M, Fulzele S. Recent advances in hyaluronic acid based therapy for osteoarthritis. Clin Transl Med. 2018;7(1):6. https://doi.org/10.1186/s40169-017-0180-3.
Pradal J, Maudens P, Gabay C, Seemayer CA, Jordan O, Allemann E. Effect of particle size on the biodistribution of nano- and microparticles following intra-articular injection in mice. Int J Pharm. 2016;498(1–2):119–29. https://doi.org/10.1016/j.ijpharm.2015.12.015.
Cao Y, Ma Y, Tao Y, Lin W, Wang P. Intra-articular drug delivery for osteoarthritis treatment. Pharmaceutics. 2021;13(12). https://doi.org/10.3390/pharmaceutics13122166.
Bhanderi M, Shah J, Gorain B, Nair AB, Jacob S, Asdaq SMB, et al. Optimized rivastigmine nanoparticles coated with Eudragit for intranasal application to brain delivery: evaluation and nasal ciliotoxicity studies. Materials (Basel). 2021;14(21). https://doi.org/10.3390/ma14216291.
Buyel JF. Statistical designs to improve downstream processing. Methods Mol Biol. 2022;2480:295–310. https://doi.org/10.1007/978-1-0716-2241-4_16.
Ya’acob A, Zainol N, Aziz NH. Application of response surface methodology for COD and ammonia removal from municipal wastewater treatment plant using acclimatized mixed culture. Heliyon. 2022;8(6): e09685. https://doi.org/10.1016/j.heliyon.2022.e09685.
Garala KC, Patel JM, Dhingani AP, Dharamsi AT. Preparation and evaluation of agglomerated crystals by crystallo-co-agglomeration: an integrated approach of principal component analysis and Box-Behnken experimental design. Int J Pharm. 2013;452(1–2):135–56. https://doi.org/10.1016/j.ijpharm.2013.04.073.
Stagner WC, Jain A, Al-Achi A, Haware RV. Employing multivariate statistics and latent variable models to identify and quantify complex relationships in typical compression studies. AAPS PharmSciTech. 2020;21(5):186. https://doi.org/10.1208/s12249-020-01712-1.
Kannappan V, Mannemala SS. Multiple response optimization of a HPLC method for the determination of enantiomeric purity of S-Ofloxacin. Chromatographia. 2014;77(17):1203–11. https://doi.org/10.1007/s10337-014-2699-4.
Kaur R, Saini S, Patel A, Sharma T, Kaur R, Katare OP, et al. Developing a validated HPLC method for quantification of Ceftazidime employing analytical quality by design and Monte Carlo simulations. J AOAC Int. 2021;104(3):620–32. https://doi.org/10.1093/jaoacint/qsab014.
Cortesi R, Esposjto E, Luca G, Nastruzzi C. Production of lipospheres as carriers for bioactive compounds. Biomaterials. 2002;23(11):2283–94. https://doi.org/10.1016/S0142-9612(01)00362-3.
Piraino LR, Benoit DSW, DeLouise LA. Optimizing soluble cues for salivary gland tissue mimetics using a design of experiments (DoE) approach. Cells. 2022;11(12):1962. https://doi.org/10.3390/cells11121962.
Katre NV, Asherman J, Schaefer H, Hora M. Multivesicular liposome (DepoFoam) technology for the sustained delivery of insulin-like growth factor-I (IGF-I). J Pharm Sci. 1998;87(11):1341–6. https://doi.org/10.1021/js980080t.
Prefilled syringes — Part 8: Requirements and test methods for finished prefilled syringes In ISO 11040-8:2016(en). https://www.iso.org/obp/ui/#iso:std:iso:11040:-8:ed-1:v1:en. Accessed 22 Aug 2022.
Prefilled syringes — Part 8: Requirements and test methods for finished prefilled syringes [Available from: https://www.iso.org/obp/ui/#iso:std:iso:11040:-8:ed-1:v1:en.
Hasandoost L, Akbarzadeh A, Attar H, Heydarinasab A. In vitro effect of imatinib mesylate loaded on polybutylcyanoacrylate nanoparticles on leukemia cell line K562. Artif Cells Nanomed Biotechnol. 2017;45(3):665–9. https://doi.org/10.1080/21691401.2016.1175444.
Esfandyari-Manesh M, Abdi M, Talasaz AH, Ebrahimi SM, Atyabi F, Dinarvand R. S2P peptide-conjugated PLGA-Maleimide-PEG nanoparticles containing Imatinib for targeting drug delivery to atherosclerotic plaques. Daru. 2020;28(1):131–8. https://doi.org/10.1007/s40199-019-00324-w.
Liu Z, Chen H, Lv F, Wang J, Zhao S, Li Y, et al. Sequential release of paclitaxel and imatinib from core-shell microparticles prepared by coaxial electrospray for vaginal therapy of cervical cancer. Int J Mol Sci. 2021;22(16). https://doi.org/10.3390/ijms22168760.
Sallam BN, Lu T, Yu H, Li Q, Sarfraz Z, Iqbal MS, et al. Productivity enhancement of cucumber (Cucumis sativus L.) through optimized use of poultry manure and mineral fertilizers under greenhouse cultivation. Horticulturae. 2021;7(8):256. https://doi.org/10.3390/horticulturae7080256.
Haware RV, Shivagari R, Johnson PR, Staton S, Stagner WC, Gupta MR. Application of multivariate methods to evaluate the functionality of bovine- and vegetable-derived magnesium stearate. J Pharm Sci. 2014;103(5):1466–77. https://doi.org/10.1002/jps.23920.
Masoudzadeh A, Alami S, Naderi Rajeh Y, Taheri E, Sadeghi H. The role of relationship emotional schemas and personality dimensions on domestic violence in people referred to a forensics center in Iran. Iran J Psychiatry. 2022;17(1):44–51. https://doi.org/10.18502/ijps.v17i1.8048.
Yoo H, Rheem I, Rheem S, Oh S. Optimizing medium components for the maximum growth of Lactobacillus plantarum JNU 2116 using response surface methodology. Korean J Food Sci Anim Resour. 2018;38(2):240–50. https://doi.org/10.5851/kosfa.2018.38.2.240.
Antonopoulou V, Goffe L, Meyer CJ, Grimani A, Graham F, Lecouturier J, et al. A comparison of seasonal influenza and novel Covid-19 vaccine intentions: a cross-sectional survey of vaccine hesitant adults in England during the 2020 pandemic. Human Vaccines & Immunotherapeutics. 2022:2085461. https://doi.org/10.1080/21645515.2022.2085461.
Dewangan HK, Pandey T, Maurya L, Singh S. Rational design and evaluation of HBsAg polymeric nanoparticles as antigen delivery carriers. Int J Biol Macromol. 2018;111:804–12. https://doi.org/10.1016/j.ijbiomac.2018.01.073.
Auwal SM, Zarei M, Tan CP, Basri M, Saari N. Enhanced physicochemical stability and efficacy of angiotensin I-converting enzyme (ACE) - inhibitory biopeptides by chitosan nanoparticles optimized using Box-Behnken design. Scientific Reports. 2018;8. https://doi.org/10.1038/s41598-018-28659-5.
Briuglia M-L, Rotella C, McFarlane A, Lamprou DA. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv Transl Res. 2015;5(3):231–42. https://doi.org/10.1007/s13346-015-0220-8.
Ahmed TA. Preparation of finasteride capsules-loaded drug nanoparticles: formulation, optimization, in vitro, and pharmacokinetic evaluation. Int J Nanomedicine. 2016;11:515–27. https://doi.org/10.2147/IJN.S98080.
Yang M, Xu B, Wang X, Li W, Cao J, Li W, et al. Effect of spray drying conditions on physical properties of Panax notoginseng Saponin (PNS) powder and the intra-batch dissolution variability of PNS hydrophilic matrix tablet. Drug Des Devel Ther. 2021;15:1425–40. https://doi.org/10.2147/DDDT.S295825.
Dinç-Zor Ş, Aksu DÖ. Box-Behnken design-desirability function approach in optimization of HPLC method for simultaneous determination of Ibuprofen along with additives in syrup formulation. J AOAC Int. 2021;104(1):78–83. https://doi.org/10.1093/jaoacint/qsaa096.
Dangre PV, Tattu AD, Borikar SP, Surana SJ, Chalikwar SS. Development and statistical optimization of alginate-Neusilin US2 micro-composite beads to elicit gastric stability and sustained action of hesperidin. Int J Biol Macromol. 2021;171:514–26. https://doi.org/10.1016/j.ijbiomac.2021.01.025.
Musazzi UM, Youm I, Murowchick JB, Ezoulin MJ, Youan BB. Resveratrol-loaded nanocarriers: formulation, optimization, characterization and in vitro toxicity on cochlear cells. Colloids Surf B Biointerfaces. 2014;118:234–42. https://doi.org/10.1016/j.colsurfb.2014.03.054.
ICH guideline Q9 on quality risk management 2015. In: ema.europa.eu, https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-3.pdf. Accessed 22 Aug 2022.
Kanda H, Katsube T, Wahyudiono, Goto M. Preparation of liposomes from soy lecithin using liquefied dimethyl ether. Foods. 2021;10(8):1789. https://doi.org/10.3390/foods10081789.
Lin X, Li B, Wen J, Wu J, Tang D, Yu Y, et al. Storage stability and in vitro bioaccessibility of liposomal betacyanins from red pitaya (Hylocereus polyrhizus). Molecules. 2022;27(4). https://doi.org/10.3390/molecules27041193.
Acknowledgements
The authors are grateful to Amneal Pharmaceuticals for facilitating the research work.
Author information
Authors and Affiliations
Contributions
Ms. Amruta designed and performed the experiments. Dr. Anita Lalwani is responsible for reviewing the manuscript, interpreting the results, and performing statistical analysis.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gorajiya, A., Lalwani, A. Leveraging the Exploratory and Predictive Capabilities of Design of Experiments in Development of Intraarticular Injection of Imatinib Mesylate Containing Lipospheres. AAPS PharmSciTech 23, 275 (2022). https://doi.org/10.1208/s12249-022-02431-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02431-5