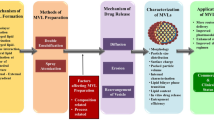
Abstract
Liposomes being a promising colloidal system facilitates delivery of drugs with limited pharmacokinetic properties to achieve desirable clinical applications. However, development of a stable liposomal system is always challenging due to multiple complexities involved. Aqueous instability of liposomes and impact of various process and formulation parameters can lead to serious alteration of its therapeutic performance. In the proposed work, the authors aim to develop stable Ibrutinib-loaded liposomes using lyophilization and Quality-by-Design and assess their long-term stability. Ibrutinib-loaded liposomes were developed and optimized using Quality-by-Design technique and were further PEGylated and characterized for the same. Effect of cryoprotectants during lyophilization and other parameters are evaluated to obtain a robust formulation. The stability studies were conducted upto 6 months at various storage conditions to evaluate the effect of lyophilization. The impact of formulation, processing and lyophilization parameters on physicochemical properties of developed liposomal systems were evaluated and are critically discussed. Liquid dispersion exhibited a %degradation of 16–36% at 25 °C/60% RH which was reduced for less than 1% in lyophilized formulation for 6 months. Critical analysis and assessment of various parameters lead to identification of optimum conditions to manufacture this drug product and also opens way forward for further evaluation and translational possibilities.
Graphical abstract








Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on request.
Abbreviations
- % EE:
-
Percent Entrapment Efficiency
- CMA:
-
Critical Material Attributes
- CPP:
-
Critical Process Parameters
- CQA:
-
Critical Quality Attributes
- DoE:
-
Design of Experiments
- DSC:
-
Differential Scanning Calorimetry
- DSPG-Na:
-
1,2-Distearoyl-sn-glycero-3-phospho-rac-glycerol, sodium salt
- FALT:
-
Fixed Aqueous Layer Thickness
- HSPC:
-
Hydrogenated Soybean Phosphatidylcholine
- IBR-LIP:
-
Ibrutinib Liposomes
- IBR-LIP-PEG:
-
Ibrutinib Liposomes PEGylated
- mPEG-2000-DSPE:
-
N-(Carbonyl-methoxypolyethylenglycol-2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine
- PS:
-
Particle Size
- QbD:
-
Quality-by-Design
- QTPP:
-
Quality Target Product Profile
- ZP:
-
Zeta Potential
References
Maja L, Željko K, Mateja P. Sustainable technologies for liposome preparation. J Supercrit Fluids. 2020;165:104984. https://doi.org/10.1016/j.supflu.2020.104984.
Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomed. 2006;1:297–315.
Mishima K. Biodegradable particle formation for drug and gene delivery using supercritical fluid and dense gas. Adv Drug Deliv Rev. 2008;60:411–32. https://doi.org/10.1016/j.addr.2007.02.003.
Wang Y, Grainger DW. Lyophilized liposome-based parenteral drug development: reviewing complex product design strategies and current regulatory environments. Adv Drug Deliv Rev. 2019;151–152:56–71. https://doi.org/10.1016/j.addr.2019.03.003.
Chen C, Han D, Cai C, Tang X. An overview of liposome lyophilization and its future potential. J Controlled Release. 2010;142:299–311. https://doi.org/10.1016/j.jconrel.2009.10.024.
Cacela C, Hincha DK. Low amounts of sucrose are sufficient to depress the phase transition temperature of Dry Phosphatidylcholine, but not for Lyoprotection of liposomes. Biophys J. 2006;90:2831–42. https://doi.org/10.1529/biophysj.105.074427.
Fonteyne M, Vercruysse J, Díaz DC, Gildemyn D, Vervaet C, Remon JP, Beer TD. Real-time assessment of critical quality attributes of a continuous granulation process. Pharm Dev Technol. 2013;18:85–97. https://doi.org/10.3109/10837450.2011.627869.
Hunter P. The fourth pillar: despite some setbacks in the clinic, immunotherapy has made notable progress toward becoming an additional therapeutic option against cancer. EMBO Rep. 2017;18:1889–92. https://doi.org/10.15252/embr.201745172.
Panchal K, Sahoo RK, Gupta U, Chaurasiya A. Role of targeted immunotherapy for pancreatic ductal adenocarcinoma (PDAC) treatment: an overview. Int Immunopharmacol. 2021;95:107508. https://doi.org/10.1016/j.intimp.2021.107508.
Paydas S. Management of adverse effects/toxicity of ibrutinib. Crit Rev Oncol Hematol. 2019;136:56–63. https://doi.org/10.1016/j.critrevonc.2019.02.001.
Profitt RT, Adler-Moore J, Chiang. S-M Amphotericin B liposome preparation.
Zhai B, Wu Q, Wang W, Zhang M, Han X, Li Q, Chen P, Chen X, Huang X, Li G, Zhang Q, Zhang R, Xiang Y, Liu S, Duan T, Lou J, Xie T, Sui X. Preparation, characterization, pharmacokinetics and anticancer effects of PEGylated β-elemene liposomes. Cancer Biol Med. 2020;17:60–75. https://doi.org/10.20892/j.issn.2095-3941.2019.0156.
Park S-J, Choo G-H, Hwang S-J, Kim M-S. Quality by design: screening of critical variables and formulation optimization of Eudragit E nanoparticles containing dutasteride. Arch Pharm Res. 2013;36:593–601. https://doi.org/10.1007/s12272-013-0064-z.
Panigrahi KC, Patra CN, Rao MEB. Quality by design enabled development of oral self-nanoemulsifying drug delivery system of a Novel Calcimimetic Cinacalcet HCl using a porous carrier: in vitro and in vivo characterisation. AAPS PharmSciTech. 2019;20:216. https://doi.org/10.1208/s12249-019-1411-2.
Lim J, Yeap SP, Che HX, Low SC. Characterization of magnetic nanoparticle by dynamic light scattering. Nanoscale Res Lett. 2013;8:381. https://doi.org/10.1186/1556-276X-8-381.
Corbett JCW, McNeil-Watson F, Jack RO, Howarth M. Measuring surface zeta potential using phase analysis light scattering in a simple dip cell arrangement. Colloids Surf Physicochem Eng Asp. 2012;396:169–76. https://doi.org/10.1016/j.colsurfa.2011.12.065.
Shimada K, Miyagishima A, Sadzuka Y, Nozawa Y, Mochizuki Y, Ohshima H, Hirota S. Determination of the thickness of the fixed aqueous layer around polyethyleneglycol-coated liposomes. J Drug Target. 1995;3:283–9. https://doi.org/10.3109/10611869509015957.
Danhier F, Lecouturier N, Vroman B, Jérôme C, Marchand-Brynaert J, Feron O, Préat V. Paclitaxel-loaded PEGylated PLGA-based nanoparticles: in vitro and in vivo evaluation. J Controlled Release. 2009;133:11–7. https://doi.org/10.1016/j.jconrel.2008.09.086.
Taylor-Vine G, Mead H, Paraskevopoulou V, Mann J. Evaluation of NanoDis as an automated sampling technology for in vitro release testing of nanomedicines. Dissolution Technol. 2023;30:126–32. https://doi.org/10.14227/DT300323P126.
Lambert WJ. Considerations in developing a Target Product Profile for Parenteral Pharmaceutical products. AAPS PharmSciTech. 2010;11:1476–81. https://doi.org/10.1208/s12249-010-9521-x.
USFDA. (2018) Liposome Drug Products: Chemistry, Manufacturing, and Controls; Human Pharmacokinetics and Bioavailability; and Labeling Documentation. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/liposome-drug-products-chemistry-manufacturing-and-controls-human-pharmacokinetics-and.
ICH PHARMACEUTICAL DEVELOPMENT Q8(R2.). https://database.ich.org/sites/default/files/Q8_R2_Guideline.pdf.
Bonde GV, Ajmal G, Yadav SK, Mittal P, Singh J, Bakde BV, Mishra B. Assessing the viability of Soluplus® self-assembled nanocolloids for sustained delivery of highly hydrophobic lapatinib (anticancer agent): optimisation and in-vitro characterisation. Colloids Surf B Biointerfaces. 2020;185:110611. https://doi.org/10.1016/j.colsurfb.2019.110611.
Lujan H, Griffin WC, Taube JH, Sayes CM. Synthesis and characterization of nanometer-sized liposomes for encapsulation and microRNA transfer to breast cancer cells. Int J Nanomed Volume. 2019;14:5159–73. https://doi.org/10.2147/IJN.S203330.
Chen H, Fang Z, Song M, Liu K. Mitochondrial targeted hierarchical drug delivery system based on HA-modified liposomes for cancer therapy. Eur J Med Chem. 2022;241:114648. https://doi.org/10.1016/j.ejmech.2022.114648.
Kong G, Braun RD, Dewhirst MW. Hyperthermia enables tumor-specific nanoparticle delivery: effect of particle size. Cancer Res. 2000;60:4440–5.
Kirby C, Gregoriadis G. Dehydration-rehydration vesicles: a simple method for High Yield Drug Entrapment in liposomes. Nat Biotechnol. 1984;2:979–84. https://doi.org/10.1038/nbt1184-979.
Sabeti B, Noordin MI, Mohd S, Hashim R, Dahlan A, Akbari Javar H. Development and characterization of liposomal doxorubicin hydrochloride with Palm Oil. BioMed Res Int. 2014;2014:1–6. https://doi.org/10.1155/2014/765426.
Smith MC, Crist RM, Clogston JD, McNeil SE. Zeta potential: a case study of cationic, anionic, and neutral liposomes. Anal Bioanal Chem. 2017;409:5779–87. https://doi.org/10.1007/s00216-017-0527-z.
Joseph E, Singhvi G. Multifunctional nanocrystals for cancer therapy: a potential nanocarrier. Nanomaterials for drug delivery and therapy. Elsevier; 2019. pp. 91–116.
StatEase Randomized Factorial Designs. https://www.statease.com/docs/v12/designs/factorial-randomized/#:~:text=Minimum%20Run%2 C%20Resolution%20IV%20Factorial,extremely%20sensitive%20to%20missing%20data.
Hu X, Wang H, Liu Y. Statistical analysis of Main and Interaction effects on Cu(II) and cr(VI) decontamination by Nitrogen–Doped magnetic graphene oxide. Sci Rep. 2016;6:34378. https://doi.org/10.1038/srep34378.
Shaker S, Gardouh A, Ghorab M. Factors affecting liposomes particle size prepared by ethanol injection method. Res Pharm Sci. 2017;12:346. https://doi.org/10.4103/1735-5362.213979.
Mukherjee S, Ray S, Thakur R. Solid lipid nanoparticles: a modern formulation approach in drug delivery system. Indian J Pharm Sci. 2009;71:349. https://doi.org/10.4103/0250-474X.57282.
He Y, Luo L, Liang S, Long M, Xu H. Influence of probe-sonication process on drug entrapment efficiency of liposomes loaded with a hydrophobic drug. Int J Polym Mater Polym Biomater. 2019;68:193–7. https://doi.org/10.1080/00914037.2018.1434651.
Shi L, Zhang J, Zhao M, Tang S, Cheng X, Zhang W, Li W, Liu X, Peng H, Wang Q. Effects of polyethylene glycol on the surface of nanoparticles for targeted drug delivery. Nanoscale. 2021;13:10748–64. https://doi.org/10.1039/D1NR02065J.
Pan Z, Fang D, Song N, Song Y, Ding M, Li J, Luo F, Tan H, Fu Q. Surface distribution and Biophysicochemical properties of Polymeric micelles Bearing Gemini Cationic and hydrophilic groups. ACS Appl Mater Interfaces. 2017;9:2138–49. https://doi.org/10.1021/acsami.6b14339.
Xu J, Gattacceca F, Amiji M. Biodistribution and Pharmacokinetics of EGFR-Targeted thiolated gelatin nanoparticles following systemic administration in pancreatic tumor-bearing mice. Mol Pharm. 2013;10:2031–44. https://doi.org/10.1021/mp400054e.
Kowalska M, Broniatowski M, Mach M, Płachta Ł, Wydro P. The effect of the polyethylene glycol chain length of a lipopolymer (DSPE-PEGn) on the properties of DPPC monolayers and bilayers. J Mol Liq. 2021;335:116529. https://doi.org/10.1016/j.molliq.2021.116529.
Ohtake S, Schebor C, Palecek SP, De Pablo JJ. Phase behavior of freeze-dried phospholipid–cholesterol mixtures stabilized with trehalose. Biochim Biophys Acta BBA - Biomembr. 2005;1713:57–64. https://doi.org/10.1016/j.bbamem.2005.05.001.
Koster KL, Lei YP, Anderson M, Martin S, Bryant G. Effects of Vitrified and Nonvitrified sugars on Phosphatidylcholine Fluid-to-gel phase transitions. Biophys J. 2000;78:1932–46. https://doi.org/10.1016/S0006-3495(00)76741-5.
Leslie SB, Israeli E, Lighthart B, Crowe JH, Crowe LM. Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl Environ Microbiol. 1995;61:3592–7. https://doi.org/10.1128/aem.61.10.3592-3597.1995.
Ohtake S, Schebor C, De Pablo JJ. Effects of trehalose on the phase behavior of DPPC–cholesterol unilamellar vesicles. Biochim Biophys Acta BBA - Biomembr. 2006;1758:65–73. https://doi.org/10.1016/j.bbamem.2006.01.002.
Yoshida K, Ono F, Chouno T, Perocho BR, Ikegami Y, Shirakigawa N, Ijima H. Cryoprotective enhancing effect of very low concentration of trehalose on the functions of primary rat hepatocytes. Regen Ther. 2020;15:173–9. https://doi.org/10.1016/j.reth.2020.08.003.
Franzé S, Selmin F, Samaritani E, Minghetti P, Cilurzo F. Lyophilization of liposomal formulations: still necessary, still challenging. Pharmaceutics. 2018;10:139. https://doi.org/10.3390/pharmaceutics10030139.
Degobert G, Aydin D. Lyophilization of Nanocapsules: instability sources, formulation and process parameters. Pharmaceutics. 2021;13:1112. https://doi.org/10.3390/pharmaceutics13081112.
Field C. Developing a Method for Suitable Loss on Drying by TGA.
Zhang M, Suo Z, Peng X, Gan N, Zhao L, Tang P, Wei X, Li H. Microcrystalline cellulose as an effective crystal growth inhibitor for the ternary Ibrutinib formulation. Carbohydr Polym. 2020;229:115476. https://doi.org/10.1016/j.carbpol.2019.115476.
Brophy MR, Deasy PB. Application of the Higuchi model for drug release from dispersed matrices to particles of general shape. Int J Pharm. 1987;37:41–7. https://doi.org/10.1016/0378-5173(87)90008-1.
USFDA Onivyde PIL. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207793lbl.pdf.
Karmakar S. Particle size distribution and Zeta potential based on dynamic light scattering: techniques to Characterise Stability and Surface distribution of Charged colloids. In: Recent Trends in Materials Physics and Chemistry; 2019.
Izutsu K-I, Yomota C, Kawanishi T. Stabilization of liposomes in Frozen Solutions through Control of Osmotic Flow and Internal Solution freezing by Trehalose. J Pharm Sci. 2011;100:2935–44. https://doi.org/10.1002/jps.22518.
Acknowledgements
The authors would also like to extend their gratitude to Central Analytical Laboratory, BITS Pilani, Hyderabad Campus for providing the necessary support.
Funding
This work was supported by the Parenteral Drug Association, India Chapter.
Author information
Authors and Affiliations
Contributions
Kanan Panchal: conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing– original draft; Akhila Reddy: investigation, methodology, writing– original draft; Rishi Paliwal: data curation, formal analysis, writing– review & editing; Akash Chaurasiya: conceptualization, funding acquisition, project administration, supervision, visualization, writing - review & editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Author declares that they have provided their consent for publishing this manuscript.
Conflict of interest
The authors have filed a patent; Indian Patent Authority, Application No. 202211003679.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Panchal, K., Reddy, A., Paliwal, R. et al. Dynamic intervention to enhance the stability of PEGylated Ibrutinib loaded lipidic nano-vesicular systems: transitioning from colloidal dispersion to lyophilized product. Drug Deliv. and Transl. Res. (2024). https://doi.org/10.1007/s13346-024-01555-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s13346-024-01555-4