Abstract
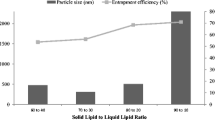
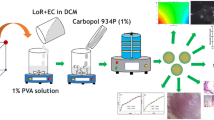
Hot melt extrusion (HME) has been used for the formulation of topical solid lipid nanoparticle (SLN) gel without using any other size reduction technique including high pressure homogenization or sonication. SLN formulation solely using HME has not been applied to other drugs except IBU. Therefore, the purpose of the present study was to formulate FLB SLN solely using HME technique and evaluate the SLN formulation in inflammation animal model. Stable 0.5% w/v FLB SLN gel with particle size < 250 nm, PI < 0.3 and EE of > 98% was prepared. Differential scanning calorimetry (DSC) thermogram showed that the drug was converted to amorphous form in the HME process. Additionally, rheological studies demonstrated that FLB SLN gel and marketed FLB gel showed shear thinning property. FLB SLN formulation showed significantly (p < 0.05) higher peak force required to spread the formulation as compared to marketed FLB formulation. Stability studies showed that FLB SLN gel was stable for a month at room temperature and 2–4°C. Moreover, in vitro permeation test (IVPT) and ex vivo skin deposition study results revealed that FLB SLN gel showed significant (p < 0.05) increase in drug deposition in dermal layer and drug permeation as compared to control marketed formulation. Further, in vivo anti-inflammatory study showed equivalent inhibition of rat paw edema using 0.5% w/v FLB SLN gel which has 10 times less strength compared to control formulation. Overall, FLB SLN formulation was successfully manufactured solely using HME technique which resulted in enhanced the skin permeation of FLB and superior anti-inflammatory activity.






Similar content being viewed by others
References
Sharadha M, et al. An overview on topical drug delivery system–updated review. International Journal of Research in Pharmaceutical Sciences. 2020;11(1):368–85.
Griffin T, et al. Potentiation of antitumor immunotoxins by liposomal monensin. JNCI: J National Cancer Inst. 1993;85(4):292–8.
Boakye CH, Patel K, Singh M. Doxorubicin liposomes as an investigative model to study the skin permeation of nanocarriers. Int J Pharm. 2015;489(1–2):106–16.
Doddapaneni R, et al. Tumor neovasculature-targeted cationic PEGylated liposomes of gambogic acid for the treatment of triple-negative breast cancer. Drug Delivery. 2016;23(4):1232–41.
Marepally S, et al. Design, synthesis of novel lipids as chemical permeation enhancers and development of nanoparticle system for transdermal drug delivery. PLoS ONE. 2013;8(12): e82581.
Patel K, et al. Tumor stromal disrupting agent enhances the anticancer efficacy of docetaxel loaded PEGylated liposomes in lung cancer. Nanomedicine. 2016;11(11):1377–92.
Patel K, et al. Combination approach of YSA peptide anchored docetaxel stealth liposomes with oral antifibrotic agent for the treatment of lung cancer. Mol Pharm. 2016;13(6):2049–58.
Godugu C, Doddapaneni R, Singh M. Honokiol nanomicellar formulation produced increased oral bioavailability and anticancer effects in triple negative breast cancer (TNBC). Colloids Surf, B. 2017;153:208–19.
Patel AR, Chougule M, Singh M. EphA2 targeting pegylated nanocarrier drug delivery system for treatment of lung cancer. Pharm Res. 2014;31(10):2796–809.
Shaik MS, Chatterjee A, Singh M. Effects of monensin liposomes on the cytotoxicity, apoptosis and expression of multidrug resistance genes in doxorubicin-resistant human breast tumour (MCF-7/dox) cell-line. J Pharm Pharmacol. 2004;56(7):899–907.
Mehnert W, Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2012;64:83–101.
Hou D, et al. The production and characteristics of solid lipid nanoparticles (SLNs). Biomaterials. 2003;24(10):1781–5.
Zoabi A, Touitou E, Margulis K. Recent advances in nanomaterials for dermal and transdermal applications. Colloids and Interfaces. 2021;5(1):18.
Scioli Montoto S, Muraca G, Ruiz ME. Solid lipid nanoparticles for drug delivery: pharmacological and biopharmaceutical aspects. Front Mol Biosci. 2020;319.
Singh M, Ferdous AJ, Jackson TL. Stealth monensin liposomes as a potentiator of adriamycin in cancer treatment. J Control Release. 1999;59(1):43–53.
Patel G, et al. Nanoliposomal dry powder formulations. Methods Enzymol. 2009;464:167–91.
Pham DT, Tran PH, Tran TT. Development of solid dispersion lipid nanoparticles for improving skin delivery. Saudi Pharmaceutical Journal. 2019;27(7):1019–24.
Passos JS, et al. Development, skin targeting and antifungal efficacy of topical lipid nanoparticles containing itraconazole. Eur J Pharm Sci. 2020;149: 105296.
Geetha T, et al. Sesamol-loaded solid lipid nanoparticles for treatment of skin cancer. J Drug Target. 2015;23(2):159–69.
Shadambikar G, et al. Formulation development of itraconazole PEGylated nano-lipid carriers for pulmonary aspergillosis using hot-melt extrusion technology. International journal of pharmaceutics: X. 2021;3: 100074.
Patil H, et al. Continuous production of fenofibrate solid lipid nanoparticles by hot-melt extrusion technology: a systematic study based on a quality by design approach. AAPS J. 2015;17(1):194–205.
Bhagurkar AM, Repka MA, Murthy SN. A novel approach for the development of a nanostructured lipid carrier formulation by hot-melt extrusion technology. J Pharm Sci. 2017;106(4):1085–91.
Bagde A, et al. Formulation of topical ibuprofen solid lipid nanoparticle (SLN) gel using hot melt extrusion technique (HME) and determining its anti-inflammatory strength. Drug Deliv Transl Res. 2019;9(4):816–27.
Fang J-Y, et al. Lipid nano/submicron emulsions as vehicles for topical flurbiprofen delivery. Drug Delivery. 2004;11(2):97–105.
Cody SL, Fegley RD, Hoy MR. Enhanced delivery of topical compositions containing flurbiprofen. Google Patents. 1998.
Czyrski, A., Determination of the lipophilicity of ibuprofen, naproxen, ketoprofen, and flurbiprofen with thin-layer chromatography. J Chemistry., 2019. 2019.
Fiume MM, et al. Safety assessment of triethanolamine and triethanolamine-containing ingredients as used in cosmetics. Int J Toxicol. 2013;32(3_suppl):59S-83S.
Kulkarni SB, Singh M, Betageri GV. Encapsulation, stability and in-vitro release characteristics of liposomal formulations of colchicine. J Pharm Pharmacol. 1997;49(5):491–5.
Chowdhury N, et al. Liposomes co-loaded with 6-phosphofructo-2-kinase/fructose-2, 6-biphosphatase 3 (PFKFB3) shRNA plasmid and docetaxel for the treatment of non-small cell lung cancer. Pharm Res. 2017;34(11):2371–84.
Affram K, et al. Smart thermosensitive liposomes for effective solid tumor therapy and in vivo imaging. PLoS ONE. 2017;12(9): e0185116.
Shaik MS, et al. Long-circulating monensin nanoparticles for the potentiation of immunotoxin and anticancer drugs. J Pharm Pharmacol. 2001;53(5):617–27.
Boakye CH, et al. Ultra-flexible nanocarriers for enhanced topical delivery of a highly lipophilic antioxidative molecule for skin cancer chemoprevention. Colloids Surf, B. 2016;143:156–67.
Akhlaq M, et al. A simple high-performance liquid chromatographic practical approach for determination of flurbiprofen. Journal of Advanced Pharmaceutical Technology & Research. 2011;2(3):151.
Pignatello R, et al. Flurbiprofen-loaded acrylate polymer nanosuspensions for ophthalmic application. Biomaterials. 2002;23(15):3247–55.
Kryscio DR, et al. Spreadability measurements to assess structural equivalence (Q3) of topical formulations—a technical note. AAPS PharmSciTech. 2008;9(1):84–6.
Salau O, Bagde A, Kalvala A, Singh M. Enhancement of transdermal permeation of cannabinoids and their pharmacodynamic evaluation in rats. Int J Pharm. 2022;624:122016.
Desai PR, et al. Dermal microdialysis technique to evaluate the trafficking of surface-modified lipid nanoparticles upon topical application. Pharm Res. 2012;29(9):2587–600.
Mallampati R, et al. Evaluation of EpiDerm full thickness-300 (EFT-300) as an in vitro model for skin irritation: studies on aliphatic hydrocarbons. Toxicol In Vitro. 2010;24(2):669–76.
Fulzele SV, et al. Estimation of proinflammatory biomarkers of skin irritation by dermal microdialysis following exposure with irritant chemicals. Toxicology. 2007;237(1–3):77–88.
Babu R, Chatterjee A, Singh M. Assessment of skin irritation and molecular responses in rat skin exposed to nonane, dodecane and tetradecane. Toxicol Lett. 2004;153(2):255–66.
Babu RJ, et al. Percutaneous absorption and anti-inflammatory effect of a substance P receptor antagonist: spantide II. Pharm Res. 2004;21(1):108–13.
Patlolla RR, et al. Dermal microdialysis of inflammatory markers induced by aliphatic hydrocarbons in rats. Toxicol Lett. 2009;185(3):168–74.
Aburahma MH, Badr-Eldin SM. Compritol 888 ATO: a multifunctional lipid excipient in drug delivery systems and nanopharmaceuticals. Expert Opin Drug Deliv. 2014;11(12):1865–83.
Yong-Tai Z, et al. Solid lipid nanoparticles formulated for transdermal aconitine administration and evaluated in vitro and in vivo. J Biomed Nanotechnol. 2015;11(2):351–61.
Hassan H, et al. Central composite design for formulation and optimization of solid lipid nanoparticles to enhance oral bioavailability of acyclovir. Molecules. 2021;26(18):5432.
Tran T, Rades T, Müllertz A. Formulation of self-nanoemulsifying drug delivery systems containing monoacyl phosphatidylcholine and Kolliphor® RH40 using experimental design. Asian J Pharm Sci. 2018;13(6):536–45.
Khan MFA, et al. Hydrogel containing solid lipid nanoparticles loaded with argan oil and simvastatin: preparation, in vitro and ex vivo assessment. Gels. 2022;8(5):277.
Deuschle VCKN, et al. Physical chemistry evaluation of stability, spreadability, in vitro antioxidant, and photo-protective capacities of topical formulations containing Calendula officinalis L. leaf extract. Brazil J Pharm Sci. 2015;51:63–75.
Patere N, S, et al. Compritol® 888 ATO a lipid excipient for sustained release of highly water soluble active: formulation, scale-up and IVIVC study. Curr Drug Deliv. 2013;10(5):548–56.
Mancini G, et al. Increased therapeutic efficacy of SLN containing etofenamate and ibuprofen in topical treatment of inflammation. Pharmaceutics. 2021;13(3):328.
Pham CV, et al. Development of ibuprofen-loaded solid lipid nanoparticle-based hydrogels for enhanced in vitro dermal permeation and in vivo topical anti-inflammatory activity. Journal of Drug Delivery Science and Technology. 2020;57: 101758.
Jain S, et al. Solid lipid nanoparticles bearing flurbiprofen for transdermal delivery. Drug Delivery. 2005;12(4):207–15.
Funding
Authors are thankful to National Institute on Minority Health and Health Disparities of National Institutes of Health, Grant/Award Number: U54 MD007582 for providing the funding for this research work.
Author information
Authors and Affiliations
Contributions
Arvind Bagde: substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work and drafting the work or revising it critically for important intellectual content.
Emmanual Kouagou: substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work.
Mandip Singh: final approval of the version to be published and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Guest Edited by Jayachandra Babu Ramapuram and Ashana Puri.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bagde, A., Kouagou, E. & Singh, M. Formulation of Topical Flurbiprofen Solid Lipid Nanoparticle Gel Formulation Using Hot Melt Extrusion Technique. AAPS PharmSciTech 23, 257 (2022). https://doi.org/10.1208/s12249-022-02410-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02410-w