Abstract
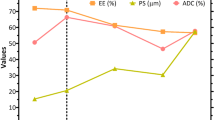
The investigation aims to prepare, identify, analyse, and understand the effects of different variables and constant factors associated with developing drug-loaded microsponge formulation by altering variables using the experiment’s design. A series of drug formulations were prepared by alteration of variables using the design of experiment (DoE). Shape factors were kept constant. Response surface methodology (RSM) was utilized to optimize the preparation and analyse factors and variables. The RSM and QbD make the process easy to scale up and reproducible to minimize batch-to-batch variation. Critical process attributes (CPAs), such as particle size, yield, and drug entrapment, were analysed and comprehended in the development process to estimate the risk of microsponge-based formulation and method stability. Critical process parameters (CPPs) were identified by trial and error. QbD-driven quasi-emulsion solvent evaporation method was adopted to emulsify the dispersed phase within a continuous aqueous phase to develop the microsponges. The investigation confirmed that the effects of changes in controlling factors were most prominent on response variables. Each response was found in a broad range in terms of average particle diameter (337–461 µm), entrapment efficiency (77.69–94.38%) of the drug, and product yield (76.91–96.38%). The optimum results are a yield of ~ 96%, an average particle size of 347 µm, and entrapment efficiency of ~ 93.55%. An analysis of ANOVA (P value < 0.05) and models were validated. A constant rate of drug release from the optimized product was observed for a period (8 h) with a drug release of 75%. The developed method is reproducible, stable, and reliable.
Graphical abstract











Similar content being viewed by others

Data Availability
There are no available data and materials.
Abbreviations
- QbD:
-
Quality by design
- CPPs:
-
Critical process parameters
- CPAs:
-
Critical process attributes
- RSM:
-
Response surface methodology
- NDDS:
-
Novel drug delivery systems
- EC-46:
-
Ethyl cellulose 46cp
- ERS-100:
-
Eudragit RS 100
- DCM:
-
Dichloromethane
- EA:
-
Ethyl alcohol
- RPM:
-
Rotation per minute
- API:
-
Active pharmaceutical ingredient
- Np:
-
Power number
- PB:
-
Phosphate buffer
- UV:
-
Ultraviolet
- DSC:
-
Differential scanning calorimetry
- FTIR:
-
Fourier transformed infrared spectroscopy
- Da:
-
Diameter of impeller
- Dt:
-
Diameter of the tank
- E:
-
Clearance of tank
- H:
-
Height of the liquid
- L:
-
Length of impeller blade
- W:
-
Width of impeller blade
- w/v:
-
Weight per volume
- cP:
-
Centipoises
- EE:
-
Entrapment efficiency
- PSD:
-
Particle size distribution
- CCD:
-
Central composite design
- ANOVA:
-
Analysis of variance
- CPF:
-
Checkpoint formulation
- CPR:
-
Cumulative percent release
- FESEM:
-
Field emission scanning electron microscope
References
Wang J, Ni Q, Wang Y, Zhang Y, He H, Gao D, Ma X, Liang XJ. Nanoscale drug delivery systems for controllable drug behaviors by multi-stage barrier penetration. J Control Release. 2021;331:282–95.
Srivastava R, Pathak K. Microsponges: a futuristic approach for oral drug delivery. Expert Opin Drug Deliv. 2012;9:863–78.
Shahzad Y, Saeed S, Ghori MU, Mahmood T, Yousaf AM, Jamshaid M, Sheikh R, Rizvi SAA. Influence of polymer ratio and surfactants on controlled drug release from cellulosic microsponges. Int J Biol Macromol. 2018;109:963–70.
Embil K, Nacht S. The Microsponge® Delivery System (MDS): a topical delivery system with reduced irritancy incorporating multiple triggering mechanisms for the release of actives. J Microencapsul. 1996;13:575–88.
Junqueira MV, Bruschi ML. A review about the drug delivery from microsponges. AAPS PharmSciTech. 2018;19:1501–11.
Singhvi G, Manchanda P, Hans N, Dubey SK, Gupta G. Microsponge: an emerging drug delivery strategy. Drug Dev Res. 2019;80:200–8.
Pawar AP, Gholap AP, Kuchekar AB, Bothiraja C, Mali AJ (2015) Formulation and Evaluation of optimized oxybenzone microsponge gel for topical delivery. J Drug Deliv 1–9.
Nacht V, Katz M (1990) A novel topical programmable delivery system. In: 1st (ed) Top. Drug Deliv. Formul., 1st ed. CRC Press, pp 299–325.
ICH Harmonised Tripartite Guideline (2009) Pharmaceutical development Q8 (R2). In: ICH Harmon. Tripart. Guidel. European Union, Japan and USA, pp 1–28.
Rogers, P. David, Krysan, Damian (2018) Chapter 61: antifungal agents. In: Laurence L. Brunton, Randa Hilal-Dandan BCK (ed) Goodman Gilman's Pharmacol. Basis Ther., 13th ed. McGraw-Hill Education, pp 1087 – 1117.
Hansch, C, Leo J A (1985) CA 91711, Log P Database. Claremont.
Ahmed S (2018) Technical and regulatory considerations for pharmaceutical product lifecycle: ICH Q12. Contract Pharma 1–31.
Gusai T, Dhavalkumar M, Soniwala M, Dudhat K, Vasoya J, Chavda J. Formulation and optimization of microsponge-loaded emulgel to improve the transdermal application of acyclovir—a DOE based approach. Drug Deliv Transl Res. 2021;11:2009–29.
Fujioka Y, Metsugi Y, Ogawara KI, Higaki K, Kimura T. Evaluation of in vivo dissolution behavior and GI transit of griseofulvin, a BCS class II drug. Int J Pharm. 2008;352:36–43.
Li SS, Li GF, Liu L, Li H, Jiang X, Li XL, Liu ZG, Zuo T, Weng LD, Liu Q. Optimization of paeonol-loaded microparticle formulation by response surface methodology. J Microencapsul. 2015;32:677–86.
Ivanova NA, Trapani A, Di FC, et al. In vitro and ex vivo studies on diltiazem hydrochloride-loaded microsponges in rectal gels for chronic anal fissures treatment. Int J Pharm. 2019;557:53–65.
Osmani RAM, Aloorkar NH, Thaware BU, Kulkarni PK, Moin A, Hani U, Srivastava A, Bhosale RR. Microsponge based drug delivery system for augmented gastroparesis therapy: formulation development and evaluation. Asian J Pharm Sci. 2015;10:442–51.
Khattab A, Nattouf A. Optimization of entrapment efficiency and release of clindamycin in microsponge based gel. Sci Rep. 2021;11:23345.
Bothiraja C, Gholap AD, Shaikh KS, Pawar AP. Investigation of ethyl cellulose microsponge gel for topical delivery of eberconazole nitrate for fungal therapy. Ther Deliv. 2014;5:781–95.
Kumar PM, Ghosh A. Development and evaluation of silver sulfadiazine loaded microsponge based gel for partial thickness (second degree) burn wounds. Eur J Pharm Sci. 2017;96:243–54.
Jain SK, Kaur M, Kalyani P, Mehra A, Kaur N, Panchal N. Microsponges enriched gel for enhanced topical delivery of 5-fluorouracil. J Microencapsul. 2019;36:677–91.
Srivastava R, Kumar D, Pathak K. Colonic luminal surface retention of meloxicam microsponges delivered by erosion based colon-targeted matrix tablet. Int J Pharm. 2012;427:153–62.
Jelvehgari M, Siahi-Shadbad MR, Azarmi S, Martin GP, Nokhodchi A. The microsponge delivery system of benzoyl peroxide: preparation, characterization and release studies. Int J Pharm. 2006;308:124–32.
Patel N, Padia N, Vadgama N, Raval M, Sheth N. Formulation and evaluation of microsponge gel for topical delivery of fluconazole for fungal therapy. J Pharm Investig. 2016;46:221–38.
Saini R, Singh SK, Verma PRP. Evaluation of carvedilol-loaded microsponges with nanometric pores using response surface methodology. J Exp Nanosci. 2014;9:831–50.
Choi MG, Kim JH. Effect of drying methods on removal of residual solvents from solvent-induced amorphous paclitaxel. Korean J Chem Eng. 2017;34:3041–7.
Van der Graaf S (2006) Membrane emulsification: droplet formation and effects of interfacial tension. Wageningen University.
Mccabe WL, Smith JC, Harriot P (1993) Unit operations of chemical engineering, 7th ed. Choice Rev Online. https://doi.org/10.5860/choice.30-6200
Witek-Krowiak A, Chojnacka K, Podstawczyk D, Dawiec A, Pokomeda K. Application of response surface methodology and artificial neural network methods in modelling and optimization of biosorption process. Bioresour Technol. 2014;160:150–60.
Li Wan Po A, Mroso PV. Drug-drug incompatibility in the solid state: kinetic interpretation, modelling and prediction. Int J Pharm. 1984;18:287–98.
IPC (2010) Indian Pharmacopoeia 2010. In: Vol. 1, 6th ed. Indian Pharmacopoeia Commission, Ghaziabad, pp 1–13.
Khalil SKH, El-Feky GS, El-Banna ST, Khalil WA. Preparation and evaluation of warfarin-β-cyclodextrin loaded chitosan nanoparticles for transdermal delivery. Carbohydr Polym. 2012;90:1244–53.
Janus E, Ossowicz P, Klebeko J, Nowak A, Duchnik W, Kucharski Ł, Klimowicz A. Enhancement of ibuprofen solubility and skin permeation by conjugation with l-valine alkyl esters. RSC Adv. 2020;10:7570–84.
Elworthy PH, Lipscomb FJ. A note on the solubility of griseofulvin. J Pharm Pharmacol. 1968;20:790–2.
Charman WN, Lai CSC, Finnin BC, Reed BL. Self-association of nicotinamide in aqueous solution: mass transport, freezing-point depression, and partition coefficient studies. Pharm Res An Off J Am Assoc Pharm Sci. 1991;98:1144–50.
Poddar S, Khanam J. Recovery of l-tryptophan from dilute aqueous feed by extractive solvent sublation and solvent extraction methods and optimization. J Mol Liq. 2022;351:11862701–12.
Poddar S, Sarat Chandra Babu J, Babu JSC, Sarat Chandra Babu J. Modelling and optimization of a pyrolysis plant using swine and goat manure as feedstock. Renew Energy. 2021;175:253–69.
Bhoop BS. Quality by design (QbD) for holistic Pharma excellence and regulatory compliance. Pharma Times. 2014;46:26–33.
Fares AR, Elmeshad AN, Kassem MAA. Enhancement of dissolution and oral bioavailability of lacidipine via pluronic P123/F127 mixed polymeric micelles: Formulation, optimization using central composite design and in vivo bioavailability study. Drug Deliv. 2018;25:132–42.
McCabe S (2018) Unit operations of chemical engineering-5th-ed. Notes Numer. Fluid Mech. Multidiscip. Des.
Tripathi PK, Gorain B, Choudhury H, Srivastava A, Kesharwani P. Dendrimer entrapped microsponge gel of dithranol for effective topical treatment. Heliyon. 2019;15:1–19.
Gahane AY, Ranjan P, Singh V, Sharma RK, Sinha N, Sharma M, Chaudhry R, Thakur AK. Fmoc-phenylalanine displays antibacterial activity against Gram-positive bacteria in gel and solution phases. Soft Matter. 2018;14:2234–44.
Sinko PJ, Singh Y (2011) Martin's physical pharmacy and pharmaceutical sciences: In: Sinko PJ (ed) Phys. Chem. Biopharm. Princ. Pharm. Sci. Sixth Ed., 6th ed. Lippincott Williams & Wilkins, pp 469–491.
Honarpour M, Koederitz L, Harvey a H, et al (2012) Bulk density and tapped Density of Powders. World Heal Organ 1–3.
Tan J, Balasubramanian BM. Particle size measurements and scanning electron microscopy (SEM) of cocoa particles refined/conched by conical and cylindrical roller stone melangers. J Food Eng. 2017;212:146–53.
Meghwal M, Goswami TK. Evaluation of size reduction and power requirement in ambient and cryogenically ground fenugreek powder. Adv Powder Technol. 2013;24:427–35.
Liu Y, Deng Y, Sun Z, Wei J, Zheng G, Asiri AM, Khan SB, Rahman MM, Zhao D. Hierarchical Cu2S microsponges constructed from nanosheets for efficient photocatalysis. Small. 2013;9:2702–8.
Lee JB, Hong J, Bonner DK, Poon Z, Hammond PT. Self-assembled RNA interference microsponges for efficient siRNA delivery. Nat Mater. 2012;11:316–22.
Çomoglu T, Gönül N, Baykara T. The effects of pressure and direct compression on tabletting of microsponges. Int J Pharm. 2002;242:191–5.
Pandit AP, Patel SA, Bhanushali VP, Kulkarni VS, Kakad VD. Nebivolol-Loaded microsponge gel for healing of diabetic wound. AAPS PharmSciTech. 2017;18:846–54.
Dash S, Murthy PN, Nath L, Chowdhury P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm - Drug Res. 2010;67:217–23.
Fu Y, Kao WJ. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin Drug Deliv. 2010;7:429–44.
Higuchi T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963;52:1145–9.
Hixson AW, Crowell JH. Dependence of reaction velocity upon surface and agitation. Ind Eng Chem. 1931;23:923–31.
Sinha Roy D, Rohera BD. Comparative evaluation of rate of hydration and matrix erosion of HEC and HPC and study of drug release from their matrices. Eur J Pharm Sci. 2002;16:193–9.
Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15:25–35.
Buntrock RE (2013) Review of the Merck Index: an encyclopedia of chemicals, drugs, and biologicals, 15th Edition. J Chem Educ 90:1115.
Felton LA (2006) A Review of: "Handbook of pharmaceutical excipients, 5th edition." Drug Dev Ind Pharm 32:1003.
Townley ER. Griseofulvin Anal Profiles Drug Subst. 1979;8:219–49.
Karmakar S, Paul S, Biswas NM, Khanam J, Kar SK, Mukherjee H, Poddar S (2020) A pharmacological audit and advancement on the recent trend of research on Ficus benghalensis L. including its in vitro hepatoprotective activity. Clin Phytoscience 6:84.
Hansch C, Leo J A (1985) Medchem Project. Claremont.
Peterson JJ, Snee RD, McAllister PR, Schofield TL, Carella AJ. Statistics in pharmaceutical development and manufacturing. J Qual Technol. 2009;41:111–34.
Natarajan U, Periyanan PR, Yang SH. Multiple-response optimization for micro-endmilling process using response surface methodology. Int J Adv Manuf Technol. 2011;56:177–85.
Montgomery DC. Design and analysis of experiments, 10th Editi. New York: Wiley; 2019.
Emami J, Hamishehkar H, Najafabadi AR, Gilani K, Minaiyan M, Mahdavi H, Mirzadeh H, Fakhari A, Nokhodchi A. Particle size design of PLGA microspheres for potential pulmonary drug delivery using response surface methodology. J Microencapsul. 2009;26:1–8.
Yang J, Feng J, Sun C, Chen W, Ma Y, Chen Z, Dong S, Deng W. Process optimization for the preparation of beta-cyhalothrin microspheres by using the response surface methodology. J Polym Environ. 2021;29:3145–53.
Das SK, Yuvaraja K, Khanam J, Nanda A. Formulation development and statistical optimization of ibuprofen-loaded polymethacrylate microspheres using response surface methodology. Chem Eng Res Des. 2015;96:1–14.
Ricci M, Giovagnoli S, Blasi P, Schoubben A, Perioli L, Rossi C. Development of liposomal capreomycin sulfate formulations: effects of formulation variables on peptide encapsulation. Int J Pharm. 2006;311:172–81.
Bhatia A, Singh B, Raza K, Wadhwa S, Katare OP. Tamoxifen-loaded lecithin organogel (LO) for topical application: development, optimization and characterization. Int J Pharm. 2013;444:47–59.
Mahesh Kumar P, Ghosh A. Development and evaluation of metronidazole loaded microsponge based gel for superficial surgical wound infections. J Drug Deliv Sci Technol. 2015;30:15–29.
Mahmoud DBED, Shukr MH, ElMeshad AN. Gastroretentive microsponge as a promising tool for prolonging the release of mitiglinide calcium in type-2 diabetes mellitus: optimization and pharmacokinetics study. AAPS PharmSciTech. 2018;19:2519–32.
Saker A, Cares-Pacheco MG, Marchal P, Falk V. Powders flowability assessment in granular compaction: what about the consistency of Hausner ratio? Powder Technol. 2019;354:52–63.
Emery E, Oliver J, Pugsley T, Sharma J, Zhou J. Flowability of moist pharmaceutical powders. Powder Technol. 2009;189:409–15.
Anderson DL, Chung-Heng C, Nacht S. Flow characteristics of loosely compacted macroporous microsponge® polymeric systems. Powder Technol. 1994;78:15–8.
Amrutiya N, Bajaj A, Madan M. Development of microsponges for topical delivery of mupirocin. AAPS PharmSciTech. 2009;10:402–9.
Nokhodchi A, Jelvehgari M, Siahi MR, Mozafari MR. Factors affecting the morphology of benzoyl peroxide microsponges. Micron. 2007;38:834–40.
Arambulo AS, Deardorff DL. Compressed tablets, average weight. J Am Pharm Assoc Am Pharm Assoc (Baltim). 1953;42:690–1.
Grabow WW, Jaeger L. SiRNA delivery: loaded-up microsponges. Nat Mater. 2012;11:268–9.
Mahant S, Kumar S, Nanda S, Rao R. Microsponges for dermatological applications: perspectives and challenges. Asian J Pharm Sci. 2020;43:469–76.
Moin A, Deb T, Osmani RM, Bhosale R, Hani U. Fabrication, characterization, and evaluation of microsponge delivery system for facilitated fungal therapy. J Basic Clin Pharm. 2016;7:39–48.
Orlu M, Cevher E, Araman A. Design and evaluation of colon specific drug delivery system containing flurbiprofen microsponges. Int J Pharm. 2006;318:103–17.
Greber G, Jeffery GH, Bassett J, Mendham J, Denney RC (1990) Vogel's textbook of quantitative chemical analysis. 5th edn. In: Endeavour, 5th ed. Longman Scientific & Technical, NEW YORK, pp 741–757.
Acknowledgements
We want to give our heartiest thanks and gratitude to the Department of Pharmaceutical Technology, Jadavpur University and the Department of Chemical Engineering, National Institute of Technology, Tiruchirappalli, Tamil Nadu. We would also like to thank Mr. Debaldeb Dutta, Mr. Sahajaman Haldar, and Dr. Ankit Jain for assisting during the experimental procedure and manuscript preparation. We would also like to acknowledge the Vice-Chancellor Prof. Dr. S. Das of Jadavpur University and Director Prof. Dr. G. Aghila, and the administration of the National Institute of Technology, Tiruchirappalli, Tamil Nadu, for helping us with immense support.
Author information
Authors and Affiliations
Contributions
Shibam Karmakar: conception, outcome curation, proper investigation, software utilization analysis, initiation and completion of the draft, and final manuscript. Sourav Poddar: conceptualization, data curation, formal analysis, investigation, software utilization, validation, visualization, and writing, review, and editing — original and final manuscript. Jasmina Khanam: conceptualization, data curation, formal analysis, investigation, software utilization, validation, visualization, and writing, review, and editing — original and final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
This article does not involve human participants, so it is not applicable. All the experimental methods were carried out according to the guidelines of the Jadavpur University, West Bengal, India. The article also follows the National Institute of Technology guidelines, Tiruchirappalli, Tamil Nadu, India.
Consent for Publication
Not applicable. However, the authors declare that no known competing financial interest or personal relationships could have appeared to influence the work reported in this manuscript.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. Statistically integrated development process with the help of design of experiment (DoE). Adoption of optimization leads to ease of scale-up and industrialization.
2. Impact assessment of the dependent and independent variables, multiple factors (shape, physical, and chemical) aggregated with the development of microsponge formulation.
3. A QbD-driven development approach for microsponge formulation associated with critical process parameters (CPPs) and critical process attributes (CPAs).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karmakar, S., Poddar, S. & Khanam, J. Understanding the Effects of Associated Factors in the Development of Microsponge-Based Drug Delivery: a Statistical Quality by Design (QbD) Approach Towards Optimization. AAPS PharmSciTech 23, 256 (2022). https://doi.org/10.1208/s12249-022-02409-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02409-3