Abstract
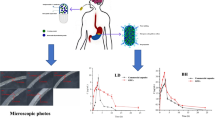
Trazodone hydrochloride (TZN) is a serotonin reuptake inhibitor that treats a major depressive disorder. It exhibits a short plasma half-life of 4.1 h and shows pH-dependent solubility. Above its pKa (6.74), solubility of TZN is very low, affecting its dissolution in the lower part of GIT. Hence, the present work aimed to develop gastro-retentive floating tablet of TZN. Central composite design was employed to optimize the formulation. Formulation variables like the concentration of HPMC-K100M, Polyox WSR 303 Leo, and sodium bicarbonate were evaluated for the responses like floating lag time and drug release. X-ray imaging study was performed on rabbits to determine the in vivo gastric retention of the optimized formulation. The accelerated stability study was conducted on optimized tablets as per ICH guidelines. Floating lag time and f2 value of the optimized formulation were found to be 2.51±0.02 min and 62.79, respectively. X-ray imaging studies in rabbits determined the in vivo gastro retention time. After 12 h of administration, tablet remained in the gastric region, indicating better retentive power. Accelerated stability studies showed sufficient formulation stability even after 3 months of storage. All these studies depict that the floating gastro-retentive system could be used as an alternative to the innovator formulation.
Graphical Abstract




Similar content being viewed by others
References
Chavanpatil MD, Jain P, Chaudhari S, Shear R, Vavia PR. Novel sustained release, swellable and bioadhesive gastroretentive drug delivery system for ofloxacin. Int J Pharm. 2006;316:86–92.
Chen Y, Ho H, Lee T, Sheu M. Physical characterizations and sustained release profiling of gastroretentive drug delivery systems with improved floating and swelling capabilities. Int J Pharm Elsevier BV. 2013;441:162–9.
Liu Q, Fassihi R. Zero-order delivery of a highly soluble , low dose drug alfuzosin hydrochloride via gastro-retentive system. Int J Pharm. 2008;348:27–34.
Eberle VA, Schoelkopf J, Gane PAC, Alles R, Huwyler J, Puchkov M. Floating gastroretentive drug delivery systems : comparison of experimental and simulated dissolution profiles and floatation behavior. Eur J Pharm Sci Elsevier BV. 2014;58:34–43.
Tang Y, Venkatraman SS, Boey FYC, Wang L. Sustained release of hydrophobic and hydrophilic drugs from a floating dosage form. Int J Pharm. 2007;336:159–65.
Shakya R, Thapa P, Saha R. In vitro and in vivo evaluation of gastroretentive floating drug delivery system of ofloxacin. Asian J Pharm Sci. 2013;8:191–8.
Stahl SM. Mechanism of action of trazodone: a multifunctional drug. CNS Spectr. 2009;14:536–46.
OleptroTM (trazodone hydrochloride) extended-release tablets. P T. 2011;36(2):2–18
Yonar D, Sünnetçioǧlu MM. Spectroscopic and calorimetric studies on trazodone hydrochloride-phosphatidylcholine liposome interactions in the presence and absence of cholesterol. Biochim Biophys Acta - Biomembr. 2014;1838:2369–79.
Gervais. US7829120B2 - Trazodone composition for once a day administration - Google Patents. United States Pat. 2010.
Gorecki D, Verbeck R. In; Florey, K. E. Analytical profile of drug substances. Academic Press Inc., London; 1987.
Elhesaisy N, Swidan S. Trazodone loaded lipid core poly (ε-caprolactone) nanocapsules: development, characterization and in vivo antidepressant effect evaluation. Sci Rep Nature Publishing Group. 2020;10:1964.
Patel M, Mori D, Dudhat K, Shah S, Chavda J, Patel A. Saturation solubility and dissolution property improvement of albendazole by salt formation approach. J Pharm Innov. Springer US; 2021;1–12. Available from: https://doi.org/10.1007/s12247-021-09588-9
Beg S, Rahman Z. Central composite designs and their applications in pharmaceutical product development. Des Exp Pharm Prod Dev. Springer, Singapore; 2021. p. 63–76.
Pharmacopeia US. USP 29–NF 24. Rockville, MD: USP; 2005.
Lachman L, Lieberman HA, JLK The theory and practice of industrial pharmacy. Third edit. Varghese Publishing House; 1987.
Jimcnez-castellanos MR, Zia H, Rhodes T. Design and testing in vitro of a bioadhesive and floating drug delivery system for oral application. Int J Pharm. 1994;105:65–70.
Pawar HA, Dhavale R. Development and evaluation of gastroretentive floating tablets of an antidepressant drug by thermoplastic granulation technique. Beni-Suef Univ J Basic Appl Sci Elsevier Ltd. 2014;3:122–32.
Shevalkar G, Pawar M, Vavia P. Nanostructured lipid carriers (NLCs) of lumefantrine with enhanced permeation. J Pharm Innov. Springer US; 2021;1–14.
Chen YC, Ho HO, Chiu CCSM. Development and characterization of a gastroretentive dosage form composed of chitosan and hydroxyethyl cellulose for alendronate. Drug Des Devel Ther. 2014;8:67–78.
Bomma R, Naidu RA, Yamsani MRVK. Development and evaluation of gastroretentive norfloxacin floating tablets. Acta Pharm. 2009;59:211–21.
Bose A, Wong TW, Singh N. Formulation development and optimization of sustained release matrix tablet of Itopride HCl by response surface methodology and its evaluation of release kinetics. Saudi Pharm J. 2013;21:201–13.
Someshwar K, Chithaluru K, Ramarao TKK. Formulation and evaluation of effervescent floating tablets of tizanidine hydrochloride. Acta Pharm. 2011;61:217–26.
Thakar K, Joshi G, Sawant KK. Bioavailability enhancement of baclofen by gastroretentive floating formulation : statistical optimization , in vitro and in vivo pharmacokinetic studies floating formulation. Drug Dev Ind Pharm. 2013;39:880–8.
Anil R, Arza K, Sekhara C, Gonugunta R, Veerareddy PR. Formulation and evaluation of swellable and floating gastroretentive ciprofloxacin hydrochloride tablets. AAPS PharmSciTech. 2009;10:220–6.
Yin L, Qin C, Chen K, Zhu C, Cao H, Zhou J, et al. Gastro-floating tablets of cephalexin : preparation and in vitro / in vivo evaluation. Int J Pharm Elsevier BV. 2013;452:241–8.
Shevalkar G, Pai R, Vavia P. Nanostructured lipid carrier of propofol: a promising alternative to marketed soybean oil–based nanoemulsion. AAPS PharmSciTech. AAPS PharmSciTech. 2019;20:1–14.
Bruschi ML. Strategies to modify the drug release from pharmaceutical systems. Woodhead Publ. 2015.
Acknowledgements
The authors are thankful to the All India Council for Technical Education (AICTE-NAFETIC) for providing research facilities. The authors are also grateful to Prof. M. M. Gatane from Bombay Veterinary College, Parel, for providing facilities to perform animal studies and Intas Pharmaceutical Pvt. Ltd., India, for giving a gift sample of trazodone hydrochloride.
Author information
Authors and Affiliations
Contributions
Manoj A. Pawar was involved in the study design, acquisition, analysis, interpretation of data, drafting, and critical revision of the manuscript.
Ganesh B. Shevalkar was involved in the analysis, interpretation of data, and manuscript drafting.
Pradeep R. Vavia was involved in the design of the study, critical revision of the manuscript, final approval of the manuscript, and corresponding author.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pawar, M.A., Shevalkar, G.B. & Vavia, P.R. Design and Development of Gastro-retentive Drug Delivery System for Trazodone Hydrochloride: a Promising Alternative to Innovator’s Controlled-Release Tablet. AAPS PharmSciTech 23, 251 (2022). https://doi.org/10.1208/s12249-022-02404-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02404-8