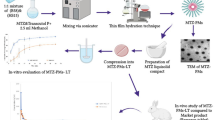
Abstract
Purpose
Sertraline HCl (SRT) is a selective serotonin reuptake inhibitor (SSRI) that is used to treat depression and anxiety disorders. SRT is characterized by limited water solubility and suffers from low bioavailability due to first-pass effect. Therefore, this study aims to formulate SRT-Cyclodextrin (CD) orodispersible tablet (ODT) using direct compression technique to avoid SRT drawbacks.
Methods
A 22 × 3 full factorial design was adopted using three factors, namely, CD type (βCD and HPβCD), SRT-CD mixing type (physical and solid dispersion), and type of diluent/superdisintegrant (xylitol/polyvinylpyrrolidone, maltitol/polyvinylpyrrolidone, and maltitol/co-processed mixture of polyvinylpyrrolidone and Ac-Di-Sol). Precompression studies were carried out on each formulation blend followed by the characterization of the prepared ODTs. All the ODTs were assessed for weight variation, hardness, friability, content uniformity, in vitro disintegration time, wetting time, water absorption ratio, in vitro dispersion time, and in vitro dissolution study. Accelerated and long-term stability studies were executed on the selected formulation. A pharmacokinetic study of the selected SRT ODT was accomplished through sublingual administration using LC/MS/MS assay in comparison to the conventional oral tablet Lustral®.
Results
ODT prepared with SRT-βCD solid dispersion with maltitol/polyvinylpyrrolidone (F4) led to reducing the disintegration time (9 ± 1.41 sec) and enhancing the dissolution efficiency (84 ± 2.5%). It showed a significant decrease of Tmax to 4 h instead of 8 h for Lustral® and increased the extent of drug absorption with a relative bioavailability of 117%.
Conclusion
SRT orodispersible sublingual tablet can be considered as a promising delivery system for the treatment of depression. SRT sublingual ODT can avoid first-pass effect of SRT and hence improve its bioavailability due to the enhancement of SRT dissolution and rapid absorption through the oral mucosa.








Similar content being viewed by others
References
World Health Organization (WHO). Depression. March 2018. https://www.who.int/news-room/fact-sheets/detail/depression.
Health NIoM. Depression. NIH Publication No. 11, p. 3561. 2011. https://www.nimh.nih.gov/health/topics/depression/index.shtml.
Kupfer DJ. Long-term treatment of depression. J Clin Psychiatr. 1991;52(Suppl):28–34.
Greenberg PE, Birnbaum HG. The economic burden of depression in the US: societal and patient perspectives. Expert Opin Pharmacother. 2005;6(3):369–76. https://doi.org/10.1517/14656566.6.3.369.
Anderson IM. Selective serotonin reuptake inhibitors versus tricyclic antidepressants: a meta-analysis of efficacy and tolerability. J Affect Disord. 2000;58(1):19–36.
Al-Nimry SS, Jaber MA. Preparation and optimization of sertraline hydrochloride tablets with improved dissolution through crystal modification. AAPS PharmSciTech. 2017;18(4):1190–202. https://doi.org/10.1208/s12249-016-0586-z.
Warrington S, Ronfeld R, Wilner K, Lazar J. Human pharmacokinetics of sertraline. Clin Neuropharmacol. 1992;15:438B.
van Harten J. Clinical pharmacokinetics of selective serotonin reuptake inhibitors. Clin Pharmacokinet. 1993;24(3):203–20. https://doi.org/10.2165/00003088-199324030-00003.
Cooper JM, Duffull SB, Saiao AS, Isbister GK. The pharmacokinetics of sertraline in overdose and the effect of activated charcoal. Br J Clin Pharmacol. 2015;79(2):307–15. https://doi.org/10.1111/bcp.12500.
Moqbel HA, ElMeshad AN, El-Nabarawi MA. A pharmaceutical study on chlorzoxazone orodispersible tablets: formulation, in-vitro and in-vivo evaluation. Drug delivery. 2016;23(8):2998–3007.
Tayel SA, El Nabarawi MA, Amin MM, AbouGhaly MH. Comparative study between different ready-made orally disintegrating platforms for the formulation of Sumatriptan succinate sublingual tablets. AAPS PharmSciTech. 2017;18(2):410–23. https://doi.org/10.1208/s12249-016-0517-z.
Russell R. Synthetic excipients challenge all-natural organics: offer advantages. Pharm Technol. 2004;28(4):38–50.
Reimerdes D. The near future of tablet excipients. Manuf Chem. 1993;64(7):14–5.
Pusapati RT, Kumar MK, Rapeti SS, Murthy T. Development of co-processed excipients in the design and evaluation of atorvastatin calcium tablets by direct compression method. Int J Pharm Investig. 2014;4(2):102–6. https://doi.org/10.4103/2230-973X.133059.
Eraga SO, Arhewoh MI, Uhumwangho MU, Iwuagwu MA. Characterisation of a novel, multifunctional, co-processed excipient and its effect on release profile of paracetamol from tablets prepared by direct compression. Asian Pac J Trop Biomed. 2015;5(9):768–72.
Abouhussein DM, Sabry P, Moghawry HM. Recent insight into critical concerns in international quality regulations governing the pharmaceutical industry: a review. Int J Pharm Res. 2019;11(1).
Loftsson T, Duchene D. Cyclodextrins and their pharmaceutical applications. Int J Pharm. 2007;329(1-2):1–11.
Ouyang H-Z, Fang L, Zhu L, Zhang L, Ren X-L, He J, et al. Effect of external factors on the curcumin/2-hydroxypropyl-β-cyclodextrin: in vitro and in vivo study. J Incl Phenom Macrocycl Chem. 2012;73(1-4):423–33.
Loh GOK, Tan YTF, Peh K-K. Enhancement of norfloxacin solubility via inclusion complexation with β-cyclodextrin and its derivative hydroxypropyl-β-cyclodextrin. Asian J Pharm Sci. 2016;11(4):536–46.
Abouhussein D, El-Bary AA, Shalaby S, El Nabarawi M. Chitosan mucoadhesive buccal films: effect of different casting solvents on their physicochemical properties. Int J Pharm Pharm Sci. 2016;8:206–13.
Khattab A, Abouhussein DM, F EM. Development of injectable tenoxicam in situ forming microparticles based on sesame oil and poly-DL-lactide: characterization, efficacy and acute toxicity. Journal of Drug Delivery Science and Technology. 2019.
Chandira M, Gosh N, Sahu S. Recent aspect of mouth dissolving tablet-an over view. J Chem Pharm Res. 2009;1(1):163–77.
Morales JO, Horng M, Gregg AM, McConville JT. Orally disintegrating tablets using starch and fructose. Pharm Technol. 2010;34(11):92–9.
Patel S, Patel M, Patel N. Flowability testing of directly compressible excipients according to British pharmacopoeia. J Pharm Res. 2009;8(2):66–9.
Ashish P, Harsoliya M, Pathan J, Shruti S. A review-formulation of mouth dissolving tablet. Int J Pharm Clin Sci. 2011;1(1):1–8.
Keleb EI, Vermeire A, Vervaet C, Remon JP. Continuous twin screw extrusion for the wet granulation of lactose. Int J Pharm. 2002;239(1-2):69–80.
Paul S, Sun CC. Dependence of friability on tablet mechanical properties and a predictive approach for binary mixtures. Pharm Res. 2017;34(12):2901–9. https://doi.org/10.1007/s11095-017-2273-5.
Moqbel HA, ElMeshad AN, El-Nabarawi MA. Comparative study of different approaches for preparation of chlorzoxazone orodispersible tablets. Drug Dev Ind Pharm. 2017;43(5):742–50. https://doi.org/10.1080/03639045.2016.1225753.
Marques MR, Loebenberg R, Almukainzi M. Simulated biological fluids with possible application in dissolution testing. Dissolution Technol. 2011;18(3):15–28.
Kim J-I, Cho S-M, Cui J-H, Cao Q-R, Oh E, Lee B-J. In vitro and in vivo correlation of disintegration and bitter taste masking using orally disintegrating tablet containing ion exchange resin-drug complex. Int J Pharm. 2013;455(1-2):31–9.
Pabari R, Ramtoola Z. Effect of a disintegration mechanism on wetting, water absorption, and disintegration time of orodispersible tablets. J Young Pharm : JYP. 2012;4(3):157–63. https://doi.org/10.4103/0975-1483.100021.
Singh J, Garg R, Gupta GD. Enhancement of solubility of lamotrigine by solid dispersion and development of orally disintegrating tablets using 3(2) full factorial design. J Pharm. 2015;2015:828453. https://doi.org/10.1155/2015/828453.
Sharma D. Formulation development and evaluation of fast disintegrating tablets of salbutamol sulphate for respiratory disorders. ISRN Pharm. 2013;2013:674507. https://doi.org/10.1155/2013/674507.
Abouhussein DMN, Bahaa El Din Mahmoud D, Mohammad FE. Design of a liquid nano-sized drug delivery system with enhanced solubility of rivaroxaban for venous thromboembolism management in paediatric patients and emergency cases. J Liposome Res. 2019:1–14. https://doi.org/10.1080/08982104.2019.1576732.
Abouhussein DM, Khattab A, Bayoumi NA, Mahmoud AF, Sakr TM. Brain targeted rivastigmine mucoadhesive thermosensitive in situ gel: optimization, in vitro evaluation, radiolabeling, in vivo pharmacokinetics and biodistribution. J Drug Deliv Sci Technol. 2018;43:129–40.
Ghaderi F, Nemati M, Siahi-Shadbad MR, Valizadeh H, Monajjemzadeh F. Physicochemical analysis and nonisothermal kinetic study of sertraline–lactose binary mixtures. J Food Drug Anal. 2017;25(3):709–16.
Sagdinc S, Kandemirli F, Bayari SH. Ab initio and density functional computations of the vibrational spectrum, molecular geometry and some molecular properties of the antidepressant drug sertraline (Zoloft) hydrochloride. Spectrochim Acta A Mol Biomol Spectrosc. 2007;66(2):405–12.
USP 41 N, (2018), , . The United States Pharmacopeial Convention, 12601, Twinbrook Parkway, Rockville, MD 20852, p. 4678. 2018.
BP. British Pharmacopeia, Tablets; Vol III-74 © Crown Copyright 2013. 2014.
Q-x L, B-z S, Yang Z-q, H-l F. Electrolytic conductivity behaviors and solution conformations of chitosan in different acid solutions. Carbohydr Polym. 2006;63(2):272–82.
Rahman Z, Zidan AS, Khan MA. Risperidone solid dispersion for orally disintegrating tablet: Its formulation design and non-destructive methods of evaluation. Int J Pharm. 2010;400(1-2):49–58.
Rezende BA, Cortes SF, De Sousa FB, Lula IS, Schmitt M, Sinisterra RD, et al. Complexation with β-cyclodextrin confers oral activity on the flavonoid dioclein. Int J Pharm. 2009;367(1-2):133–9.
Bala R, Khanna S, Pawar P. Polymers in fast disintegrating tablets–a review. Asian J Pharm Clin Res. 2012;5(2):8–14.
Aldawsari H, Altaf A, Banjar Z, Okubo M, Iohara D, Anraku M, et al. Combined use of cyclodextrins and Hydroxypropylmethylcellulose Stearoxy Ether (Sangelose®) for the preparation of orally disintegrating tablets of type-2 antidiabetes agent glimepiride. J Incl Phenom Macrocycl Chem. 2014;80(1-2):61–7.
Ishiguro T, Morishita E, Iohara D, Hirayama F, Wada K, Motoyama K, et al. Some pharmaceutical and inclusion properties of 2-hydroxybutyl-β-cyclodextrin derivative. Int J Pharm. 2011;419(1-2):161–9.
Ikeda Y, Motoune S, Ono M, Arima H, Hirayama F, Uekama K. Potential use of 2-hydroxypropyl-β-cyclodextrin as a release modifier of a water-soluble drug, metoprolol tartrate, from ethylcellulose tablets. Journal of Drug Delivery Science and Technology. 2004;14(1):69–76.
Shoukri RA, Ahmed IS, Shamma RN. In vitro and in vivo evaluation of nimesulide lyophilized orally disintegrating tablets. Eur J Pharm Biopharm. 2009;73(1):162–71.
Kuno Y, Kojima M, Ando S, Nakagami H. Evaluation of rapidly disintegrating tablets manufactured by phase transition of sugar alcohols. J Control Release. 2005;105(1-2):16–22.
Jambhekar S, Casella R, Maher T. The physicochemical characteristics and bioavailability of indomethacin from β-cyclodextrin, hydroxyethyl-β-cyclodextrin, and hydroxypropyl-β-cyclodextrin complexes. Int J Pharm. 2004;270(1-2):149–66.
Acknowledgment
Authors would like to acknowledge the National Organization for Drug Control and Research (NODCAR) for providing all the necessary facilities during the experimental work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Declaration of Interest
The authors declare that they have no conflict(s) of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abouhussein, D.M.N., El Nabarawi, M.A., Shalaby, S.H. et al. Sertraline-Cyclodextrin Complex Orodispersible Sublingual Tablet: Optimization, Stability, and Pharmacokinetics. J Pharm Innov 16, 53–66 (2021). https://doi.org/10.1007/s12247-019-09416-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-019-09416-1