Abstract
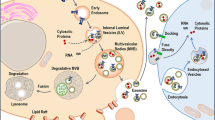
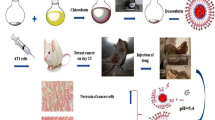
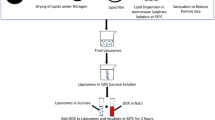
Doxorubicin (DOX) is a chemotherapeutic agent that has been used in the treatment of breast cancer. However, serious toxic effects have limited its use, mainly cardiotoxicity. To minimize the adverse effects, liposomal preparations containing DOX have been developed. These preparations can reach the target in the tumor region as well as bypass the resistance-related problems. An alternative to increased therapeutic efficacy may be the fusion of liposomes with exosomes released from tumor cells to facilitate membrane and fusion interactions, achieving greater cell uptake. Thus, the purpose of this study was the fusion of exosomes derived from breast tumor cells with long-circulating and pH-sensitive liposomes loading DOX (ExoSpHL-DOX) for the treatment of breast cancer. The mean diameter of ExoSpHL-DOX was 100.8 ± 7.8 nm, the polydispersity index was 0.122 ± 0.004, and the encapsulated DOX content was equal to 83.5 ± 2.5%. The fusion of exosomes with long-circulating and pH-sensitive liposomes was confirmed by Fourier transform infrared spectroscopy, Raman spectroscopy, and nano-flow cytometry. The physicochemical characteristics of ExoSpHL-DOX were maintained for 60 days, at 4 °C. The study of the release of DOX from ExoSpHL-DOX in dilution media with different pH values showed the pH sensitivity characteristic of the nanosystem, since 96.6 ± 0.2% of DOX was released from ExoSpHL-DOX at pH 5.0, while at pH 7.4, the release was 70.1 ± 1.7% in the medium. The cytotoxic study against the breast cancer cell line demonstrated that ExoSpHL-DOX treatment significantly reduced the cancer cell viability.
Graphical Abstract








Similar content being viewed by others
References
American Cancer Society (ACS): Cancer facts and figures 2020. Available in: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2020.html. Accessed on April 20, 2021.
Zhao N, Woodle MC, Mixson AJ. Advances in delivery systems for doxorubicin. J Nanomed Nanotechnol. 2018;9(5):519. https://doi.org/10.4172/2157-7439.1000519.
Kizek R, et al. Anthracyclines and ellipticines as DNA-damaging anticancer drugs: recent advances. Pharmacol Ther. 2012;133:26–39.
Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 2013;65(2):157–70.
Yang T, et al. Enhanced solubility and stability of PEGylated liposomal paclitaxel: in vitro and in vivo evaluation. Int J Pharm. 2007;338:317–26.
Núñez C, et al. An overview of the effective combination therapies for the treatment of breast cancer. Biomaterials. 2016;97:34–50.
Ngan YH, Gupta M. A comparison between liposomal and nonliposomal formulations of doxorubicin in the treatment of cancer: an updated review. Arch Pharm Pract. 2016;7:1–13.
Gabizon A, et al. Dose dependency of pharmacokinetics and therapeutic efficacy of pegylated liposomal doxorubicin (DOXIL) in murine models. J Drug Target. 2002;10(7):539–48.
Tardi PG, Boman NL, Cullis PR. Liposomal doxorubicin. J Drug Target. 1996;4(3):129–40.
Silva JO, et al. Folate-coated, long-circulating and pH-sensitive liposomes enhance doxorubicin antitumor effect in a breast cancer animal model. Biomed Pharmacother. 2019;118:109323.
Bunggulawa EJ. Recent advancements in the use of exosomes as drug delivery systems. J Nanobiotechnol. 2018;16(81):1–13.
Gyorgy B, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68(16):2667–88.
Kim MS, et al. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomedicine. 2018;14(1):195–204.
Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965;13(1):238–52.
Ferreira DS, et al. Development of a bone-targeted pH-sensitive liposomal formulation: physicochemical characterization, cytotoxicity, and biodistribution evaluation in a mouse model of bone metastasis. Int J Nanomed. 2016;11:3737–51.
Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1(3):1112–6. https://doi.org/10.1038/nprot.2006.179.
Faried M, et al. Membrane surface-enhanced Raman spectroscopy for cholesterol modified lipid systems: effect of gold nanoparticle size. ACS Omega. 2019;4:13687–95.
Fox CB, Uibel FH, Harris JM. Detecting phase transitions in phosphatidylcholine vesicles by Raman microscopy and self-modeling curve resolution. J Phys Chem B. 2007;111:11428–36.
Kruglik SG, et al. Raman tweezers microspectroscopy of circa 100 nm extracellular vesicles. Nanoscale. 2019;11:1661–79.
Lopes WA, Fascio M. Esquema para interpretação de espectros de substâncias orgânicas na região do infravermelho. Quim Nova. 2004;27(4):670–3.
Mihály J, et al. Characterization of extracellular vesicles by IR spectroscopy: fast and simple classification based on amide and CH stretching vibrations. Biochim Biophys Acta Biomembr. 2016;1859(3):459–166.
Hurvitz AZ, et al. FTIR-based spectrum of salivary exosomes coupled with computational-aided discriminating analysis in the diagnosis of oral cancer. J Cancer Res Clin Oncol. 2019;145(3):685–94.
Martins TS, et al. Potential of FTIR spectroscopy applied to exosomes for Alzheimer’s disease discrimination: a pilot study. J Alzheimer’s Dis. 2020;74:391–405.
Tang YT, et al. Comparison of isolation methods of exosomes and exosomal RNA from cell culture medium and serum. Int J Mol Med. 2017;40:834–44.
Gurunathan S, et al. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. 2019;8(4):307.
Lv Q, et al. Thermosensitive exosome–liposome hybrid nanoparticle-mediated chemoimmunotherapy for improved treatment of metastatic peritoneal cancer. Adv Sci. 2020;7(18):2000515.
Wang K. et al. An exosome-like programmable-bioactivating paclitaxel prodrug nanoplatform for enhanced breast cancer metastasis inhibition. Biomaterials. 2020;257.
Nie H, et al. Use of lung-specific exosomes for miRNA-126 delivery in non-small cell lung cancer. Nanoscale. 2020;12:877–87.
Li L, et al. Exosome-liposome hybrid nanoparticle codelivery of TP and miR497 conspicuously overcomes chemoresistant ovarian cancer. J Nanobiotechnol. 2022;20:50. https://doi.org/10.1186/s12951-022-01264-5.
Franco MS, et al. Triggered drug release from liposomes: exploiting the outer and inner tumor environment. Front Oncol. 2021;11:623760. https://doi.org/10.3389/fonc.2021.623760.
Gomes ER, et al. Long-circulating and fusogenic liposomes loaded with a glucoevatromonoside derivative induce potent antitumor response. Biomed Pharmacother. 2018;108:1152–61.
Novais MVM, et al. Liposomes co-encapsulating doxorubicin and glucoevatromonoside derivative induce synergic cytotoxic response against breast cancer cell lines. Biomed Pharmacother. 2021;136:111123. https://doi.org/10.1016/j.biopha.2020.111123.
Silva JO, et al. Toxicological study of a new doxorubicin-loaded pH-sensitive liposome: a preclinical approach. Toxicol Appl Pharmacol. 2018;352:162–9.
Padda RS, et al. Nanoscale flow cytometry to distinguish subpopulations of prostate extracellular vesicles in patient plasma. Prostate. 2019;79(6):592–603.
Acknowledgements
We thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the scholarship provided to Eliza Rocha Gomes.
Funding
This work was supported by the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) (PPM-00703–18, CDS-APQ-01588–15, and CDS-RED-00007–14-Rede Mineira de Pesquisas em Nanobiotecnologia) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (306197/2014–6 and 307098/2018–4).
Author information
Authors and Affiliations
Contributions
ERG designed and performed the analysis, collected the data, interpreted all the studies, and wrote the article; ATC performed the analysis, collected the data, and interpreted the nano-flow cytometry studies; TCB, LLF, and HDRC performed the analysis, collected the data, and interpreted the Raman and Fourier transform infrared spectroscopy; APS and MCF were responsible for revising the article for intellectual content. All the authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gomes, E.R., Carvalho, A.T., Barbosa, T.C. et al. Fusion of Tumor-Derived Exosomes with Long-Circulating and pH-Sensitive Liposomes Loaded with Doxorubicin for the Treatment of Breast Cancer. AAPS PharmSciTech 23, 255 (2022). https://doi.org/10.1208/s12249-022-02349-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02349-y