Abstract
Self-nanoemulsifying drug delivery systems (SNEDDS) represent an interesting platform for improving the oral bioavailability of poorly soluble lipophilic drugs. While Liquid-SNEDDS (L-SNEDDS) effectively solubilize the drug in vivo, they have several drawbacks, including poor storage stability. Solid-SNEDDS (S-SNEDDS) combine the advantages of L-SNEDDS with those of solid dosage forms, particularly stability. The aim of the present study was to convert celecoxib L-SNEDDS into S-SNEDDS without altering their release behavior. Various commercially available adsorptive carrier materials were investigated, as well as novel cellulose-based microparticles prepared by spray drying from an aqueous dispersion containing Diacel® 10 and methyl cellulose or gum arabic as a binder prior to their use. Particle size and morphology of the carrier materials were screened by scanning electron microscopy and their effects on the loading capacity for L-SNEDDS were investigated, and comparative in vitro dissolution studies of celecoxib L-SNEDDS and the different S-SNEDDS were performed immediately after preparation and after 3 months of storage. Among the adsorptive carrier materials, the novel cellulose-based microparticles were found to be the most suitable for the preparation of celecoxib S-SNEDDS from L-SNEDDS, enabling the preparation of a solid, stable formulation while preserving the in vitro release performance of the L-SNEDDS formulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many new drug candidates are characterized by poor water solubility and for this reason often have poor oral bioavailability [1]. Since this trend will certainly continue in the coming decades, the question of how to overcome this limitation is becoming more and more important. Lipid-based drug delivery systems, particularly self-nanoemulsifying drug delivery system (SNEDDS), represent an interesting platform for improving the oral bioavailability of drugs that are poorly soluble in aqueous media but can be well absorbed through the intestinal wall. SNEDDS are made from a combination of different excipients, such as lipids/oils, surfactants and co-solvents and typically present liquid formulations [2,3,4]. Oral administration of SNEDDS aims to generate a transparent emulsion characterized by an average droplet size of approximately 100 nm upon contact with gastrointestinal fluids at conditions of average gastrointestinal motility [5]. If poorly soluble drugs are formulated in Liquid-SNEDDS (L-SNEDDS), they usually remain dissolved in the inner phase of the resulting emulsion and are solubilized in this way. Currently, several L-SNEDDS formulations are available on the market [1]. However, while L-SNEDDS, once a suitable excipient combination for a given drug has been determined, are relatively quick and easy to prepare and effectively solubilize the drug in vivo, they also have some major drawbacks [6]. These arise, among other factors, from the need for complex processes for their further conversion into suitable dosage forms, such as the filling of L-SNEDDS into soft capsules, or sealed hard capsules, which are often associated with high production costs [2, 6, 7]. In addition, these liquid formulations often have low storage stability as well as limited shelf life [2, 6,7,8]. Moreover, they pose the risk of interactions with the capsule shell material, leakage from the capsule, and are hardly suitable for the development of controlled release dosage forms [2, 9]. Conversion of L-SNEDDS into Solid-SNEDDS (S-SNEDDS) could be a method of overcoming these drawbacks. Adsorption onto a solid carrier represents a suitable technology for this purpose and various materials with different chemical as well as biological origins can be utilized as adsorptive carriers [2, 10,11,12,13,14].
Due to their different chemical compositions and the methods used for their manufacture, adsorptive carrier materials may differ in terms of many of their properties, such as particle size and particle size distribution, surface area, morphology, density, porosity, plasticity, hardness, acid–base resistance, polarity, and the potential for molecular interactions [10, 11, 15]. These material-specific properties can affect the loading capacity for liquid formulations, further processing (e.g., capsule filling or tableting) and drug release [15,16,17,18]. The selection of the right carrier should allow the design of an individual, tailor-made formulation depending on the desired properties of the S-SNEDDS to be produced. By carefully combining specific carrier materials and properties with L-SNEDDS, it would ideally be possible to produce tailor-made S-SNEDDS with predefined properties. Materials that can be considered as solid carriers comprise silicon dioxide, calcium silicate, other inorganic magnesium and calcium salts, cellulose or starch, and their derivatives [2, 10, 12, 13, 19,20,21].
When L-SNEDDS are adsorbed onto a specific adsorptive carrier material, it is important to maintain their good release behavior, which can be a problem for the obtained S-SNEDDS formulation [14, 22, 23]. For example, studies by Patki et al. [23], in which the drug release of fenofibrate S-SNEDDS prepared from L-SNEDDS of this poorly water-soluble drug and various carrier materials, showed a decrease in drug release after adsorption to a solid carrier compared to fenofibrate L-SNEDDS. Although the release studies were conducted in a surfactant-containing medium (900 ml distilled water with 0.75% sodium lauryl sulfate (SLS) added), complete fenofibrate release did not occur, indicating limited drug desorption with respect to the carrier materials used. Carrier materials used in this study comprised hydroxypropyl methyl cellulose (HPMC), calcium silicate (FLORITE®), polyvinyl alcohol (PVA), magnesium aluminometasilicate (Neusilin® US2), amorphous anhydrous colloidal silica (AEROSIL® 200), and mixtures thereof. Similarly, a decreased celecoxib release was reported by Chavan et al. [14], when studying drug release of celecoxib L-SNEDDS in 900 ml of distilled water before and after adsorption onto silica-based carrier materials. Finally, also Cerpnjak et al. [22], who used maltodextrin as well as HPMC as adsorptive carriers for celecoxib L-SNEDDS, observed incomplete drug release after adsorbing L-SNEDDS to a solid carrier. Thus, the advantages of converting L-SNEDDS into solid formulations have initially proven to be only theoretical in most cases. The identification of suitable solid carrier materials that can be loaded with L-SNEDDS allows a high loading capacity and does not significantly impair the release of the adsorbed L-SNEDDS formulation or the contained active ingredient remains a challenge.
Although a variety of adsorptive carriers is already available, there is still room for new materials when it comes to overcoming the existing limitations of carriers in the field of L-SNEDDS conversion by adsorption technologies. The aim of the present study was to convert celecoxib L-SNEDDS developed in a previous study by Schmied et al. [24] into S-SNEDDS without altering their release behavior. Various commercially available adsorptive carrier materials as well as a novel biodegradable cellulose-based microparticle material should be evaluated for their suitability for S-SNEDDS preparation. Particles with a spherical, porous design should first be produced from the new cellulose-based material to enable good processability and the highest possible loading capacity for L-SNEDDS. To investigate the influence of material and particle characteristics of the different adsorptive carriers on drug release, particle size and morphology and their effects on the loading capacity for L-SNEDDS should be screened. Furthermore, comparative in vitro dissolution studies of the celecoxib L-SNEDDS and S-SNEDDS should be performed.
Materials and Methods
Materials
Celecoxib was obtained from Aarti Drugs Ltd. (Mumbai, India). Methyl cellulose, polyoxyethylene (80) sorbitan monooleate (Tween® 80), and d-α-tocopherol polyethylene glycol 1000 succinate (d-TPGS) were purchased from Sigma Aldrich Chemie GmbH (Steinheim, Germany). Gelucire® 44/14 was kindly donated by Gattefossé S.A.S (Saint Priest, France). Miglyol® 812 was purchased from Caesar & Loretz GmbH (Hilden, Germany). Aeroperl® 300 Pharma, Zeopharm® 600, and Sipernat® 160 PQ were in-house products of Evonik Resource Efficiency GmbH (Hanau, Germany). Syloid® XDP 3050 was kindly donated by W. R. Grace & Co.-Conn. (Worms, Germany). Avicel® PH-101 was obtained from Dow Chemical Company (Schwalbach am Taunus, Germany) and Diacel® 10 was purchased from CFF GmbH & Co. KG (Gehren, Germany). Gum arabic was obtained from Norevo GmbH (Hamburg, Germany). All other chemicals and solvents were of analytical grade and purchased commercially.
Methods
L-SNEDDS
Celecoxib L-SNEDDS were prepared as described by Schmied et al. [24] in a previous study based on solubility studies of the specific drug in a variety of excipients selected from lipids, (co)surfactants, and (co)solvents (data not shown). Combining the application of a systematic triangular design approach [24] and multiple analytical methods resulted in the selected celecoxib L-SNEDDS formulations, which were then used for subsequent, further processing into S-SNEDDS. The best-performing excipient composition and mixing ratio for L-SNEDDS were derived from meeting the following self-imposed specifications: the resulting nanoemulsion after dispersion of the L-SNEDDS in water had to have a droplet size < 50 nm, a polydispersity index (PDI) < 0.15, and a transmittance of > 99% (data not shown) [24]. The formulation used for all adsorption experiments in the present study consisted of 30.27% (w/w) Miglyol® 812, 49.85% (w/w) Tween® 80, 4.55% (w/w) Gelucire® 44/14, 6.24% (w/w) d-TPGS, and 9.09% (w/w) celecoxib.
Manufacturing of Cellulose-Based Microparticles as Adsorptive Carrier
Cellulose microparticles were made from native Diacel® 10 cellulose material by spray drying using an aqueous dispersion containing Diacel® 10 and methyl cellulose (MC) or gum arabic (GA) as a binder. The quantities of cellulose, binder, and deionized water were chosen in order to obtain a 10 or 20% (w/w) solid concentration in the dispersion and a 9:1 ratio (w/w) of cellulose to binder in the final dry product. Dispersions were made as follows: first, the binders were dissolved in water. For obtaining a gum arabic solution, gum arabic was stepwise added to the water while stirring with an overhead stirrer at 600 rpm. After completing the addition of gum arabic, stirring was continued for 30 min until a clear solution was obtained. The methyl cellulose colloidal solution was made by heating half of the total water quantity to 80°C before stepwise adding methyl cellulose while stirring as described for gum arabic. The second half of the total water volume was kept at room temperature, then added to the methyl cellulose dispersion, and the mixture was stirred for another 40 min at 600 rpm. Thereafter, while stirring (600 rpm), the Diacel® 10 cellulose material was added to the binder solution and stirring was continued for another 30 min at 600 rpm. Subsequently, the resulting aqueous cellulose dispersion was spray dried using a Niro Minor spray dryer (GEA Group AG, Düsseldorf, Germany). For this purpose, the dispersion was fed into the nozzle at a flow rate of 2.5 kg/h via a tube connected to a peristaltic pump. The dispersion was atomized into fine droplets using a two-fluid nozzle with a bore diameter of 1 mm. Nitrogen was used as the atomizing gas and the temperature of the drying air at the inlet was 200°C. The process was operated in the top-spray mode and the dry particles, referred to as microcellulose-methyl cellulose particles (M-MC) or microcellulose-gum arabic particles (M-GA), were collected in a vessel after being separated from the gas stream via a cyclone.
Particle Size Distribution Analysis of Adsorptive Carriers by Laser Diffraction
All adsorptive carrier materials applied in the study, i.e., calcium silicate (Zeopharm® 600), silicon dioxide (Aeroperl® 300 Pharma, Sipernat® 160 PQ, Syloid® XDP 3050), and cellulose (Avicel® PH-101, M-MC, and M-GA), were subject to investigation of particle size distribution by laser diffraction analysis using a Mastersizer 3000 from Malvern Panalytical Ltd. (Malvern, UK). The particles were suspended in Milli Q water and the sample concentration was guided by the measured obscuration. All samples were analyzed in an obscuration range of 5–10% to ensure an optimal signal to noise ratio and the average particle size d50 of individual samples was determined in triplicate.
Morphology Analysis of Adsorptive Carriers by Scanning Electron Microscopy (SEM)
To get an estimate of the morphology of the solid carrier materials, a representative sample of particles, approximately 30 mg, was sputtered with gold in an argon plasma for 30 s at 40 mA in a Jeol JFC-1300 auto fine coater from Nikon Metrology GmbH (Alzenau, Germany). Subsequently, microscopic images of the particles were taken using the Jeol Neoscope JCM-5000 scanning electron microscope from Nikon Metrology GmbH (Alzenau, Germany) at an accelerating voltage of 10 kV.
Preparation of S-SNEDDS
Celecoxib S-SNEDDS were prepared by adsorption of anhydrous celecoxib L-SNEDDS onto the individual solid carrier materials. For this purpose, 1.0 g of the liquid phase, i.e., the celecoxib L-SNEDDS formulation comprising a combination of lipids/oils, surfactants, co-solvents, and the dissolved drug substance, was placed in a 25-ml glass beaker under moderate stirring using a propeller stirrer at 100 rpm. With stirring, a suitable amount of adsorptive material was added to the liquid phase in small portions, depending on the properties of the carrier material. In order to achieve as reproducible an end point as possible, the approximate amount of carrier material needed to adsorb this amount of L-SNEDDS had been determined in a previous step for each individual formulation using the same procedure. The adsorption process was considered complete once a dry, solid, and flowable material was obtained. The end point of the adsorption process and the flowability properties were determined visually. In the present case, the end point was reached when the formulation did not stick to either the wall of the beaker or the propeller stirrer used for mixing. The flowable material obtained was then stirred for an additional 2 min at 100 rpm to ensure the formation of a homogenous S-SNEDDS formulation. In this way, three individual S-SNEDDS formulations were prepared for each adsorptive carrier.
Based on the amount of solid carrier material required for solidifying L-SNEDDS, the loading capacity, expressed as loading factor, for each carrier was determined according to the following equation:
where the calculation of the loading factor for the adsorptive carrier materials is based on L-SNEDDS.
HPLC Method for Celecoxib Quantification
High-performance liquid chromatography (HPLC) was used for celecoxib quantification [24]. The HPLC system (Agilent 1260 Infinity) consisted of a quaternary pump (G1311B), autosampler (G1329B), column oven (G1316A), and UV detector (G1314C), all from Agilent Technologies (Frankfurt am Main, Germany). Separation was achieved using a Knauer Nucleosil 100–7 C18 (125 × 4.6 mm, 7 μm) column maintained at 40°C. The mobile phase consisted of an acetonitrile:water:triethylamine mixture (300:300:0.9 v/v), adjusted to pH 3.0 with phosphoric acid. The flow rate was set to 1.8 ml/min. An injection volume of 5 μl was applied, run time was 7 min, and celecoxib was detected at 254 nm. In the concentration range of 0.13–542 μg/ml, the analytical curve was linear (r2 = 0.999995). The method was found to be accurate (100.2–102.1%) and precise (CV 2.46%) with a quantification limit of 0.05 μg/ml. Selectivity was determined (formulation excipients) and no interference was observed in drug retention time. Moreover, the celecoxib peak area did not change in the presence of all excipients used in the study.
Drug Load of Celecoxib S-SNEDDS
To determine the drug load of each of the celecoxib S-SNEDDS formulations, a defined quantity of 10.0 mg of celecoxib S-SNEDDS was transferred to a 25-ml volumetric flask, diluted with approximately 20 ml of the mobile phase described in the section “HPLC method for analyzing celecoxib,” and subjected to an intense ultrasonic treatment for 10 min. The volumetric flask was then filled with mobile phase to the calibration mark (25.0 ml) and the contents were mixed by manual shaking. Subsequently, 1.0 ml of the resulting mixture was transferred to a small safe-lock tube and centrifuged for 1 min at 9300 relative centrifugation forces (rcf). The supernatant of the centrifugate was transferred into an HPLC vial and analyzed via HPLC. All investigations were performed in triplicate and the actual drug load of the S-SNEDDS was calculated from the celecoxib concentration measured in the sample and the S-SNEDDS weight used for analysis. Using the calculated drug load, the yield of the loading process was also calculated by relating the actual and theoretical drug load.
Differential Scanning Calorimetry Analysis
All celecoxib loaded S-SNEDDSs were analyzed via differential scanning calorimetry (DSC) to determine whether the incorporated drug was in the amorphous or crystalline state. All DSC analyses were conducted using a DSC 3+ (DSC-HC01) from Mettler Toledo (Giessen, Germany). A sample of 5–10 mg each was weighed into a small, aluminum pan with a perforated lid, and exposed to a heating–cooling-heating cycle in a temperature range of 0 to 200°C. The heating/cooling rate was set at 10°C/min and a nitrogen flow of 50 ml/min was applied while running the measurement. For comparison, the melting point of the pure drug substance was investigated. For all analyzed samples, the information on the amorphous or crystalline state was taken from the thermogram obtained from the first heating cycle. Each sample was prepared and analyzed in triplicate.
Dissolution Testing of Celecoxib SNEDDS
Dissolution experiments were performed with 25 mg celecoxib or an equivalent amount of L-/S-SNEDDS using USP apparatus II (DT 800 LH) from ERWEKA GmbH (Langen, Germany). The paddle speed was set to 100 rpm to avoid coning effects and the experiments were performed in 500 ml of 0.1 N hydrochloric acid (HCl) as well as in 500 ml of phosphate buffer 6.8 USP, both maintained at 37 ± 0.5°C. Samples were withdrawn via a fraction collector, equipped with poroplast cannula filters with a pore size of 10 μm, and were then diluted 1+1 (v/v) with acetonitrile and analyzed by HPLC.
Stability Studies
Quantities of approximately 5 g of L-/S-SNEDDS were added to a 30-ml amber glass jars, closed with a screw cap, and stored at constant and controlled conditions (30°C/65% RH) in a climatic chamber from Binder GmbH (Tuttlingen, Germany) for 3 months. After 3 months, they were again subjected to dissolution experiments and the results of these tests were compared with those obtained immediately after manufacture.
Results and Discussion
Particle Size Distribution and Morphology of the Adsorptive Carriers
Results from the particle size analysis determination are summarized in Table 1. Most of the carrier materials were characterized by a particle size (d50) in the lower micrometer range. With a d50 of 8 ± 1 μm, the Zeopharm® 600 calcium silicate particles exhibited the smallest particle size, whereas the largest particle size (d50 = 182 ± 7) was measured for the novel M-GA particles.
SEM studies of all adsorptive carrier materials were performed at different magnification levels to analyze whether differences in surface area, morphology, and porosity could be identified. Aeroperl® 300 Pharma (Figs. 1a and 2a) presented with a smooth surface and a donut-like shape while Zeopharm® 600 (Figs. 1b and 2b) and Sipernat® 160 PQ (Figs. 1c and 2c) demonstrated a coarse surface and a flake-like structure with smaller particle agglomerates. Syloid® XDP 3050 (Figs. 1d and 2d) showed a structure of differently sized blocks with a smooth surface. None of the silica-based carriers investigated in this study exhibited a microporous surface, but a mesoporous texture resulting in nanopores (pore size 1–50 nm). Avicel® PH-101 (Figs. 1e and 2e) presented with elongated flake-like fragments of different sizes. The novel cellulose-based microparticles M-MC (Figs. 1f and 2f) and M-GA (Figs. 1g and 2g) exhibited distinctly different particle morphologies. In the case of M-MC, a mixture of spherical and flake-like particles as well as elongated, fibrous structures was identified. In contrast to M-MC, M-GA exhibited spherical particles with a highly porous surface, i.e., the particle structure targeted in this study. Interestingly, the replacement of the binder MC with GA in the preparation of the M-MC and M-GA particles had a significant impact on the morphology and size of the particles, although the same process parameters were used for the preparation of these microparticles. These observations are in accordance with those reported by other researchers, e.g., Alhassan et al. [25] who reported that depending on its concentration used in a formulation gum arabic may tremendously affect mechanical properties such as tensile strength as well as the modulus of elasticity when combined with a polymer. Furthermore, as reported by several research groups, also the viscosity of the spray dispersion either prepared with gum arabic or methyl cellulose may have an impact on the obtained particles’ morphology and size [25, 26].
S-SNEDDS Manufacture and Characterization
The L-SNEDDS formulation selected for the manufacturing of S-SNEDDS had been established in a previous study [24] and consisted of 30.27% (w/w) Miglyol® 812, 49.85% (w/w) Tween® 80, 4.55% (w/w) Gelucire® 44/14, 6.24% (w/w) d-TPGS, and 9.09% (w/w) celecoxib. In the cited study, this formulation provided excellent emulsification properties resulting in small droplet sizes and a narrow PDI [24]. Moreover, an initial set of dissolution experiments had indicated fast and complete drug release in conditions of the fasted stomach. In the present study, this L-SNEDDS formulation was prepared and adsorbed to a solid carrier material applying a gentle preparation method protecting the formulations from high temperatures and strong mechanical forces. Mechanisms that are, or can be, involved in such an adsorption process include capillary forces, a synergistic effect of the surface tension of L-SNEDDS, and the interfacial tension between L-SNEDDS and the capillaries of the carrier, and intermolecular adhesion forces triggered by the formation of covalent bonds, hydrogen bonds, dipole–dipole, electrostatic, and hydrophobic interactions when L-SNEDDS and the porous carrier material are combined [20, 21]. L-SNEDDS adsorption to the solid carriers provided powders with (visually assessed) varying flow properties, depending on the carriers’ properties.
The evaluation of the loading factors for the adsorptive carrier materials revealed that Sipernat® 160 PQ as well as Zeopharm® 600 exhibited the highest capacity for L-SNEDDS adsorption and consequently presented the highest drug load (Table 1). These two carriers also demonstrated the smallest particle size of all carriers analyzed (Table 1). This observation suggests that a smaller particle size, which correlates with a larger surface area, may be associated with a higher loading capacity in the case of the silica-based materials. The loading capacity of all cellulose-based carriers was lower than that of silicon-based materials, but the loading capacity of both M-GA and M-MC was higher than that of the commercial Avicel® PH-101, although the Avicel® PH-101 particles were slightly smaller than M-MC particles and significantly smaller than M-GA particles (Table 1). These results underline that not just the average particle diameter, but also the surface area and porosity of the cellulose-based materials are crucial factors for their loading capacity. M-MC may compensate for its lower porosity compared to M-GA by its much smaller particle size and/or its mixture of particles of different morphologies. Interestingly, replacing the binder MC with GA in the preparation of the cellulose-based microparticles M-MC and M-GA revealed to have a tremendous impact both on morphology and size of the particles.
As for the loading capacity, drug load was similar for M-MC (4.86 ± 0.04%) and M-GA (4.84 ± 0.02%). Overall, drug load of the S-SNEDDS correlated with the loading capacity and was highest for the Zeopharm® 600 calcium silicate particles (6.39 ± 0.01%) and lowest for the Avicel® PH-101 particles (4.31 ± 0.06%). Regardless of the substrate, the yield of the S-SNEDDS preparation was consistently above 98.5%, indicating an effective manufacturing process.
DSC analysis (thermograms displayed in endo up mode) of the celecoxib S-SNEDDS (Fig. 3), as well as the corresponding pure drug substance, indicated amorphous properties (no melting peaks) for all celecoxib S-SNEDDS (Fig. 3), while the crystalline character of the pure drug was demonstrated by a characteristic peak in the corresponding melting range (162–164°C) of the celecoxib (Fig. 3). These results show that the adsorption process of celecoxib-containing L-SNEDDS onto solid carriers, regardless of the carrier material used, had no effect on the amorphicity of celecoxib.
Dissolution Studies of L-/S-SNEDDS
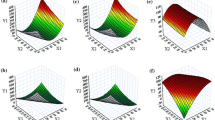
A celecoxib dose of 25 mg or an equivalent amount of L-/S-SNEDDS was screened in the dissolution experiments. Results of the release experiments performed in 0.1 N HCl are shown in Fig. 4. Both L-SNEDDS and S-SNEDDS formulations showed a rapid release, i.e., release profiles reached a plateau within the first 15 min of the experiment and no precipitation was observed until the end of the experiment (120 min). Indicative of the poor solubility of celecoxib, dissolution of the pure drug was poor with less than 5% of the tested dose dissolved at the end of the experiment. Whereas a rapid drug release was observed for all SNEDDS formulations, the fraction of dose released differed significantly among the formulations tested. The celecoxib L-SNEDDS as such showed a release of about 96% in 15 min and the release profile of the L-SNEDDS did not noticeably change after preparing S-SNEDDS by adsorption to M-MC and M-GA. Celecoxib S-SNEDDS made with Avicel® PH-101 also revealed a rapid and high drug release with approximately 90% of the dose released within in the first 15 min of the experiment. By contrast, drug release of all other S-SNEDDS formulations was incomplete. Within the test duration of 120 min, S-SNEDDS made of Sipernat® 160 PQ and Zeopharm® 600 showed a total drug release of about 60%. With a release of 35% and 25% of the dose applied, an even lower release was observed for celecoxib in the experiments with S-SNEDDS containing Aeroperl® 300 Pharma and Syloid® XDP 3050, respectively. These results indicate that silica-based carrier materials retained a proportionally larger fraction of the adsorbed drug formulation than did the cellulose-based carrier materials. Based on these observations, it can be assumed that molecular interactions between the adsorbed drug formulation and the carrier material can have a great impact on the equilibrium between adsorption and desorption processes and thus may have a strong influence on drug release.
Initial screening experiments were performed in 0.1 N HCl. Therefore, the results would indicate whether and to what extent the formulations would release under conditions of fasting stomach. To screen, whether the best-performing S-SNEDDS would also release in small intestinal pH conditions, an additional set of experiments was performed in phosphate buffer pH 6.8 USP. This second set of experiments comprised dissolution studies with cellulose-based celecoxib S-SNEDDS, celecoxib L-SNEDDS, and celecoxib drug substance. As can be seen from Fig. 5, for all formulations tested, dissolution profiles obtained in phosphate buffer pH 6.8 were like those in 0.1 N HCl indicating that that adsorption of L-SNEDDS onto M-MC and M-GA did not affect the original release performance of the L-SNEDDS formulation and also indicate that L-SNEDDS or drug release was not affected by pH conditions that may occur in the fasting stomach and mid-small intestine, respectively.
The challenge of releasing a self-emulsifying drug release system from an adsorptive solid carrier material without significantly affecting drug release compared to its liquid state has already been reported from other studies. Chavan et al. [14] studied drug release from silica-based celecoxib S-SEDDS containing 100 mg celecoxib in 900 ml distilled water in USP apparatus II at 200 rpm. Whereas drug release of the L-SNEDDS formulation was complete after 120 min, that of the S-SNEDDS did not exceed 10% of the tested dose for most of the carrier materials within the same test duration. Only for one formulation, a release of 40% of the tested dose could be observed in the same time frame. Results of the cited study thus show into the same direction as those from the present study; however, it should be noted that the silica-based materials used by Chavan et al. [14] were different from those applied in the present study, that the drug load of the tested S-SNEDDS was slightly higher and, that also a higher celecoxib dose (100 mg vs. 25 mg) was studied in the dissolution experiments.
Another study assessing the impact of adsorbing self-emulsifying formulations to solid carriers on drug release was published by Cerpnjak et al. [22], who adsorbed naproxen liquid self-microemulsifying drug delivery systems (L-SMEDDS) to maltodextrin, hypromellose, and a combination of the two as a solid carrier and studied drug release of the obtained solid SMEDDS (S-SMEDDS) in 900 ml of 0.1 N hydrochloric acid in USP apparatus II at 100 rpm. The drug load of the S-SMEDDS was 6%. Whereas one of the S-SMEDDS formulations tested preserved the self-microemulsifying properties of L-SMEDDS and exhibited dissolution profiles similar to those of L-SMEDDS, the others did not. Overall, results of the study of Cerpnjak et al. [22] also confirm the hypothesis that there are several factors that determine the drug release of solid self-emulsifying formulations, including the underlying L-SNEDDS formulation, the material, and a number of other characteristics of the solid carrier. In addition, it should be noted that the drug loads of the formulations in the cited and the present study were similar, but naproxen had a significantly better solubility in the release medium than celecoxib. In the naproxen study, also a higher media volume was used, but nevertheless complete drug release could not be achieved with each of the carrier materials. This underlines the results of the present study, especially those of the dissolution studies with M-MC- and M-GA-based celecoxib S-SNEDDS.
None of the carrier materials used in the present study was soluble in the test medium, leading to the assumption that the release of the drug was solely caused by the detachment of L-SNEDDS from the surface of the carrier and/or its removal from the carriers’ pores. Due to these preconditions, the release of active substances seems to be predominantly determined by complex material- and morphology-dependent adsorption and desorption processes, in which not only simple monolayer molecular adsorption, but also effects such as capillary condensation can play a role, especially in mesoporous systems. Differences in the extent and rate of desorption can accordingly have a direct impact on the release characteristics of the formulation and explain the different release profiles obtained in the present study.
Stability Studies of L-/S-SNEDDS
After storing L-/S-SNEDDS at 30°C/65% RH for 3 months, dissolution studies were again performed in 0.1 N HCl and in phosphate buffer pH 6.8. Results are shown in Figs. 6 and 7. In both dissolution media, celecoxib release profiles were similar to those recorded immediately after manufacture of L-SNEDDS and S-SNEDDS. After a total test duration of 120 min, the maximum difference between the total amount of celecoxib release immediately after preparation and that after 3 months of storage was < 1.5% for all formulations tested, which proves the stability of the release mechanism over the storage period.
In summary, the results of the study clearly indicate that of the carrier materials investigated, the cellulose-based microparticles M-MC and M-GA are the most suitable for the production of celecoxib S-SNEDDS from L-SNEDDS, as they allow the preparation of a solid, stable formulation while preserving the in vitro release performance of the L-SNEDDS formulation. In the present study, celecoxib was used as a model drug. However, the tested dose was in the non-therapeutic range. Future studies will focus on other poorly soluble drugs whose therapeutic dose is in the investigated dose range, as well as on significantly increasing the drug loading of L-SNEDDS and S-SNEDDS so that they can be used for higher-dose poorly soluble drugs without the need to divide a single dose among multiple dosage forms.
Conclusion
With suitable adsorptive carrier materials, it is possible to formulate drug-loaded L-SNEDDS into S-SNEDDS without affecting the rate and extent of drug release of the L-SNEDDS used. As anticipated, both the carrier material and the particle size and morphology have a crucial impact on the success of this formulation approach. In the present study, a microparticulate carrier based on cellulose and gum arabic which was prepared by spray drying was found to be the most promising candidate among all carrier materials evaluated. This novel adsorptive carrier exhibited the desired spherical particle design, which is essential for good processability and reproducible drug loading and enabled a good drug release performance. The results of the present study give rise to further investigations to evaluate the suitability of the carrier material for loading with other L-SNEDDS, especially those with higher drug contents, to further improve the carrier material in terms of particle size and morphology if necessary, and ultimately to provide an alternative approach for the formulation of poorly soluble drugs.
References
Kalepu S, Nekkanti V. Insoluble drug delivery strategies: review of recent advances and business prospects. Acta Pharm Sin B. 2015;5(5):442–53. https://doi.org/10.1016/j.apsb.2015.07.003.
Dokania S, Joshi AK. Self-microemulsifying drug delivery system (SMEDDS) - challenges and road ahead. Drug Deliv. 2015;22(6):675–90. https://doi.org/10.3109/10717544.2014.896058.
Porter CJH, Pouton CW, Cuine JF, Charman WN. Enhancing intestinal drug solubilisation using lipid-based delivery systems. Adv Drug Deliv Rev. 2008;60(6):673–91. https://doi.org/10.1016/j.addr.2007.10.014.
Pouton CW. Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and ‘self-microemulsifying’ drug delivery systems. Eur J Pharm Sci. 2000;11(2):93–8. https://doi.org/10.1016/s0928-0987(00)00167-6.
Thomas N, Holm R, Mullertz A, Rades T. In vitro and in vivo performance of novel supersaturated self-nanoemulsifying drug delivery systems (super-SNEDDS). J Control Release. 2012;160(1):25–32. https://doi.org/10.1016/j.jconrel.2012.02.027.
Vithani K, Hawley A, Jannin V, Pouton C, Boyd BJ. Inclusion of digestible surfactants in solid SMEDDS formulation removes lag time and influences the formation of structured particles during digestion. AAPS J. 2017;19(3):754–64. https://doi.org/10.1208/s12248-016-0036-6.
Chatterjee B, Almurisi SH, Dukhan AAM, Mandal UK, Sengupta P. Controversies with self-emulsifying drug delivery system from pharmacokinetic point of view. Drug Deliv. 2016;23(9):3639–52. https://doi.org/10.1080/10717544.2016.1214990.
Yetukuri K, Sudheer P. Approaches to development of solid - self micron emulsifying drug delivery system: formulation techniques an dosage forms: A review. Int J Pharm Sci Res. 2012;3(10):3550–8.
Vithani K, Hawley A, Jannin V, Pouton C, Boyd BJ. Solubilisation behaviour of poorly water-soluble drugs during digestion of solid SMEDDS. Eur J Pharm Biopharm. 2018;130:236–46. https://doi.org/10.1016/j.ejpb.2018.07.006.
Hanada M, Jermain SV, Williams RO III. Enhanced dissolution of a porous carrier-containing ternary amorphous solid dispersion system prepared by a hot melt method. J Pharm Sci. 2018;107:362–71. https://doi.org/10.1016/j.xphs.2017.09.025.
Kim DS, Yang ES, Yong CS, Youn YS, Oh KT, Li DX, et al. Effect of inorganic mesoporous carriers on 1-palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol-loaded solid self-emulsifying drug delivery system: physicochemical characterization and bioavailability in rats. Colloids Surf B Biointerfaces. 2017;160:331–6. https://doi.org/10.1016/j.colsurfb.2017.09.041.
Milović M, Djuriš J, Djekić L, Vasiljević D, Ibrić S. Characterization and evaluation of solid self-microemulsifying drug delivery systems with porous carriers as systems for improved carbamazepine release. Int J Pharm. 2012;436(1–2):58–65. https://doi.org/10.1016/j.ijpharm.2012.06.032.
Sander C, Holm P. Porous magnesium aluminometasilicate tablets as carrier of a cyclosporine self-emulsifying formulation. AAPS PharmSciTech. 2009;10(4):1388–95. https://doi.org/10.1208/s12249-009-9340-0.
Chavan RB, Modi SR, Bansal AK. Role of solid carriers in pharmaceutical performance of solid supersaturable SEDDS of celecoxib. Int J Pharm. 2015;495(1):374–84. https://doi.org/10.1016/j.ijpharm.2015.09.011.
Yeom DW, Chae BR, Kim JH, Chae JS, Shin DJ, Kim CH, et al. Solid formulation of a supersaturable self-microemulsifying drug delivery system for valsartan with improved dissolution and bioavailability. Oncotarget. 2017;8(55):94297–316. https://doi.org/10.18632/oncotarget.21691.
Hu X, Lin C, Chen D, Zhang J, Liu Z, Wu W, et al. Sirolimus solid self-microemulsifying pellets: formulation development, characterization and bioavailability evaluation. Int J Pharm. 2012;438:123–33. https://doi.org/10.1016/j.ijpharm.2012.07.055.
Sohn Y, Lee SY, Lee GH, Na YJ, Kim SY, Seong I, et al. Development of self-microemulsifying bilayer tablets for pH-independent fast release of candesartan cilexetil. Pharmazie. 2012;67:917–24.
Tung NT, Tran CS, Pham TMH, Nguyen HA, Nguyen TL, Chi SC, et al. Development of solidified self-microemulsifying drug delivery systems containing l-tetrahydropalmatine: design of experiment approach and bioavailability comparison. Int J Pharm. 2018;537:9–21. https://doi.org/10.1016/j.ijpharm.2017.12.027.
Borkar N, Xia D, Holm R, Gan Y, Mullertz A, Yang M, et al. Investigating the correlation between in vivo absorption and in vitro release of fenofibrate from lipid matrix particles in biorelevant medium. Eur J Pharm Sci. 2014;51:204–10. https://doi.org/10.1016/j.ejps.2013.09.022.
Li T, Chen X, Liu Y, Fan L, Lin L, Xu Y, et al. pH-Sensitive mesoporous silica nanoparticles anticancer prodrugs for sustained release of ursolic acid and the enhanced anti-cancer efficacy for hepatocellular carcinoma cancer. Eur J Pharm Sci. 2017;96:456–63. https://doi.org/10.1016/j.ejps.2016.10.019.
Stjern L, Voittonen S, Weldemichel R, Thuresson S, Agnes M, Benkovics G, et al. Cyclodextrin-mesoporous silica particle composites for controlled antibiotic release A proof of concept toward colon targeting. Int J Pharm. 2017;531:595–605. https://doi.org/10.1016/j.ijpharm.2017.05.062.
Čerpnjak K, Zvonar A, Vrečer F, Gašperlin M. Characterization of naproxen-loaded solid SMEDDSs prepared by spray drying: The effect of the polysaccharide carrier and naproxen. Int J Pharm. 2015;485:215–28. https://doi.org/10.1016/j.ijpharm.2015.03.015.
Patki M, Patel K. Development of a solid supersaturated self nanoemulsifying preconcentrate (S-superSNEP) of fenofibrate using dimethylacetamide and a novel co-processed excipient. Drug Dev Ind Pharm. 2018;45(3):405–14. https://doi.org/10.1080/03639045.2018.1546311.
Schmied FP, Bernhardt A, Engel A, Klein S. A customized screening tool approach for the development of a self-nanoemulsifying drug delivery system (SNEDDS). AAPS PharmSciTech. 2022;23(1):39. https://doi.org/10.1208/s12249-021-02176-7.
Alhassan SI, Mamza PAP, Ja’oAM. Effect of pure and modified gum arabic on the mechanical properties of poly (vinyl chloride). IJSRP. 2015;5(5):1–7.
Nasatto PL, Pignon F, Silveira JLM, Duarte MER, Noseda MD, Rinaudo M. Interfacial properties of methylcelluloses: the influence of molar mass. Polymers (Basel). 2014;6:2961–73. https://doi.org/10.3390/polym6122961.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors declare that this research received no specific funding, grants or sponsorship from any funding agency in the public or not-for-profit sectors. Fabian-Pascal Schmied is an industrial PhD student employed by Evonik Operations GmbH, Research, Development & Innovation, Darmstadt, Germany.
Author information
Authors and Affiliations
Contributions
Fabian-Pascal Schmied: methodology, validation, formal analysis, investigation, data curation, writing—original draft, visualization; Alexander Bernhardt: methodology, formal analysis, resources, writing—review and editing, supervision, project administration; Victor Baudron: methodology, investigation, resources; Birte Beine: data curation, resources, project administration; Sandra Klein: methodology, resources, writing—review and editing, supervision, project administration.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schmied, FP., Bernhardt, A., Baudron, V. et al. Development and Characterization of Celecoxib Solid Self-nanoemulsifying Drug Delivery Systems (S-SNEDDS) Prepared Using Novel Cellulose-Based Microparticles as Adsorptive Carriers. AAPS PharmSciTech 23, 213 (2022). https://doi.org/10.1208/s12249-022-02347-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02347-0