Abstract
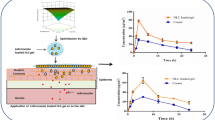
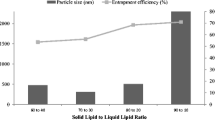
Skin ulcers have increased sharply due to rise in the incidence of obesity and diabetes. This study investigated lipid nanocarriers as a strategy to improve the efficacy of levofloxacin (LV) in penetrating skin. Two surfactant types and different lipid mixtures were used in preparation of lipid nanocarriers. Mean particle size, percentage entrapment efficiency (%EE), in vitro release, and antimicrobial activity were examined. The selected formula was incorporated into a chitosan (CS) film that was subjected to physic-chemical characterization and ex vivo permeation study. The selected formula showed particle size, PDI, and ZP: 80.3 nm, −0.21, and −26 mV, respectively, synchronized with 82.12 %EE. In vitro release study showed slow biphasic release of LV from lipid nanocarriers. The antimicrobial effect illustrated statistically significant effect of lipid nanocarriers on decreasing the minimum effective concentration (MIC) of LV, particularly against E. coli. The optimized nanocarriers’ formula loaded into CS film was clear, colorless, translucent, and smooth in texture. Based on the release profiles, it could be speculated that the CS film loaded with LV nanocarriers can maintain the antibacterial activity for 4 consecutive days. Thus, the local delivery of the drug in a sustained release manner could be predicted to enhance the therapeutic effect. Further clinical studies are strongly recommended.

Graphical Abstract





Similar content being viewed by others
References
Wang W, Lu KJ, Yu CH, Huang QL, Du YZ. Nano-drug delivery systems in wound treatment and skin regeneration. J Nanobiotechnology. 2019;17(1):82.
Lopez-Cervantes J, Escárcega-Galaz AA, SánchezMachado DI, De La Cruz-Mercado JL, Perez-Gómez LE, Ornelas-Aguirre JM. Characterization and efficacy of chitosan membranes in the treatment of skin ulcers. Egypt J Basic Appl Sci. 2019;6(1):195–205.
Westby MJ, Dumville JC, Soares MO, Stubbs N, Norman G. Dressings and topical agents for treating pressure ulcers. Cochrane Database Syst Rev. 2017;6(6):CD011947.
Gangawane AK, Bhatt B, Sunmeet M. Skin infections in diabetes: a review. J Diabetes Metab. 2016;7:644.
Negut I, Grumezescu V, Grumezescu AM. Treatment strategies for infected wounds. Molecules. 2018;23(9):2392.
Pompilio A, De Nicola S, Crocetta V, Guarnieri S, Savini V, Carretto E, et al. New insights in Staphylococcus pseudintermedius pathogenicity: antibiotic-resistant biofilm formation by a human wound-associated strain. BMC Microbiol. 2015;15:109.
Westgate SJ, Cutting K. The role of microbial biofilms in chronic and acute wound. Nursing and Residental Care. 2011;11:518–21.
López-López M, Fernández-Delgado A, Moyá ML, Blanco-Arévalo D, Carrera C, De la Haba RR, et al. Optimized preparation of levofloxacin loaded polymeric nanoparticles. Pharmaceutics. 2019;11(2):57.
Zafer A, Imam SS, Bukhari SN, Ahmad J, Ali A. Formulation and optimization of levofloxacin loaded chitosan nanoparticles for ocular delivery: in vitro characterization, ocular tolerance and antibacterial activity. Int J Biol Macromol. 2018;108:650–9.
Kumar G, Sharma S, Shafiq N, Khuller GK, Malhotra S. Optimization, in vitro–in vivo evaluation, and short-term tolerability of novel levofloxacin-loaded PLGA nanoparticle formulation. J Pharm Sci. 2012;101(6):2165–76.
Jalvandi J, White M, Gao Y, Truong YB, Padhye R, Kyratzis IL. Slow release of levofloxacin conjugated on silica nanoparticles from poly(ε-caprolactone) nanofibers Int. J Polym Mater. 2017;66:1–8.
Paladini F, Pollini M. Antimicrobial silver nanoparticles for wound healing application: progress and future trends. Materials. 2019;12(16):2540.
Adair JH, Parette MP, Altinoglu EI, Kester M. Nanoparticulate alternatives for drug delivery. ACS Nano. 2010;4(9):4967–70.
Pelgrift RY, Friedman AJ. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv Drug Deliv Rev. 2013;6:1803–15.
Kalhapure RS, Suleman N, Mocktar C, Seedast N, Govender T. Nanoengineered drug delivery systems for enhancing antibiotic therapy. J Pharm Sci. 2015;104(3):872–905.
Chang CH, Lin YH, Yeh CL, Chen YC, Chiou SF, Hsu YM, Chen YS, Wang CC. Nanoparticles Incorporated in pH-sensitive hydrogels as amoxicillin delivery for eradication of Helicobacter pylori. Biomacromolecules. 2010;11(1):133–42.
Radovic-Moreno AF, Lu TK, Puscasu VA, Yoon CJ, Langer R, Farokhzad OC. Surface charge-switching polymeric nanoparticles for bacterial cell wall-targeted delivery of antibiotics. ACS Nano. 2012;6(5):4279–87.
Jain D, Banerjee R. Comparison of ciprofloxacin hydrochloride-loaded protein, lipid, and chitosan nanoparticles for drug delivery. J Biomed Mater Res B Appl Biomater. 2008;86(1):105–12.
Pardeike J, Hommoss A, Müller RH. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int J Pharm. 2009;366(1-2):170–84.
Zsikó S, Cutcher K, Kovács A, Budai-Szűcs M, Gácsi A, Baki G, Csányi E, Berkó S. Nanostructured lipid carrier gel for the dermal application of lidocaine: comparison of skin penetration testing methods. Pharmaceutics. 2019;11(7):310.
Muller RH, Keck CM. Challenges and solutions for the delivery of biotech drugs - a review of drug nanocrystal technology and lipid nanoparticles. J Biotechnol. 2004;113(1-3):151–70.
Jenning V, Schäfer-Korting M, Gohla S. Vitamin A-loaded solid lipid nanoparticles for topical use: drug release properties. J Control Release. 2000;66(2-3):115–26.
Müller RH, Petersen RD, Hommoss A, Pardeike J. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Adv Drug Deliv Rev. 2007;59(6):522–30.
Sanna V, Caria G, Mariani A. Effect of lipid nanoparticles containing fatty alcohols having different chain length on the ex vivo skin permeability of Econazole nitrate. Powder Technol. 2010;201:32–6.
Puglia C, Bonina F. Lipid nanoparticles as novel delivery systems for cosmetics and dermal pharmaceuticals. Expert Opin on Drug Deliv. 2012;9(4):429–41.
El-Leithy ES, Abdel-Rashid RS. Lipid nanocarriers for Tamoxifen citrate/coenzyme Q10 dual delivery. J Drug Deliv Sci Technol. 2017;41:239–50.
Giovino C, Ayensu I, Tetteh J, Boateng JS. Development and characterization of chitosan films impregnated with insulin loaded PEG-b-PLA nanoparticles (NPs): a potential approach for buccal delivery of macromolecules. Int J Pharm. 2012;428(1-2):143–51.
Castro PM, Fonte P, Oliveira A, Madureira R, Sarmento B, Pintado ME. Optimization of two biopolymer-based oral films for the delivery of bioactive molecules. Mater Sci Eng C Mater Biol Appl. 2017;76:171–80.
El-Leithy ES, Shaker DS, Ghorab MK, Abdel-Rashid RS. Evaluation of mucoadhesive hydrogels loaded with diclofenac sodium–chitosan microspheres for rectal administration. AAPS Pharm SciTech. 2010;11(4):1695–702.
Wang JJ, Zeng ZW, Xiao RZ, Xie T, Zhou GL, Zhan XR, Wang SL. Recent advances of chitosan nanoparticles as drug carriers. Int J Nanomedicine. 2011;6:765–74.
Sadiq AA, Abdul Rassol AA. Formulation and evaluation of silibinin loaded solid lipid nanoparticles for peroral use targeting lower part of gastrointestinal tract. Int J Pharm Pharm Sci. 2014;6:55–67.
Jankie S, Johnson J, Adebayo AS, Pillai GK, Pereira LMP. Efficacy of levofloxacin loaded nonionic surfactant vesicles (niosomes) in a model of Pseudomonas aeruginosa infected Sprague Dawley rats. Adv Pharmacol Pharm Sci. 2020;2020:1–7.
Siafaka P, Okur ME, Ayla S, Er S, Cağlar ES, Okur NU. Design and characterization of nanocarriers loaded with Levofloxacin for enhanced antimicrobial activity; physicochemical properties, in vitro release and oral acute toxicity. Braz J Pharm Sci. 2019;55:1–13.
Batista P, Castro P, Madureira AR, Sarmento B, Pintado M. Development and characterization of chitosan microparticles-in-films for buccal delivery of bioactive peptides. Pharmaceuticals (Basel). 2019;12(1):32.
Naik S, Raikar P, Ahmed MG. Formulation and evaluation of chitosan films containing sparfloxacin for the treatment of periodontitis. J Drug Deliv Ther. 2019;9:38–45.
Parhi R, Suresh P. Formulation optimization and characterization of transdermal film of simvastatin by response surface methodology. Mater Sci Eng C Mater Biol Appl. 2016;58:331–41.
Rani S, Singh N. Formulation and characterization of periodontal films containing Azithromycin and Serratiopeptidase. Asian J Pharm Clin Res. 2018;11:205–9.
Bahri-Najafi R, Tavakoli N, Senemar M, Peikanpour M. Preparation and pharmaceutical evaluation of glibenclamide slow release mucoadhesive buccal film. Res Pharm Sci. 2014;9(3):213–23.
Neupane R, Boddu SHS, Renukuntla J, Babu RJ, Tiwari AK. Alternatives to biological skin in permeation studies: current trends and possibilities. Pharmaceutics. 2020;12(2):152.
Nasr M, Younes H, Abdel-Rashid RS. Formulation and evaluation of cubosomes containing colchicine for transdermal delivery. Drug Deliv Transl Res. 2020;10(5):1302–13.
Gonzalez-Mira E, Egea MA, Garcia ML, Souto EB. Design and ocular tolerance of flurbiprofen loaded ultrasound-engineered NLC. Colloids Surf B: Biointerfaces. 2010;81(2):412–21.
Kelidari HR, Moazeni M, Babaei R, Saeedi M, Akbari J, Parkoohi PI, Nabili M, Gohar AA, Morteza-Semnani K, Nokhodchi A. Improved yeast delivery of fluconazole with a nanostructured lipid carrier system. Biomed Pharmacother. 2017;89:83–8.
Montoto SS, Muraca G, Ruiz ME. Solid lipid nanoparticles for drug delivery: pharmacological and biopharmaceutical aspects. Front Mol Biosci. 2020;7:587997.
El Leithy ES, Abdel-Bar HM, Ali RA. Folate-chitosan nanoparticles triggered insulin cellular uptake and improved in vivo hypoglycemic activity. Int J Pharm. 2019;571:118708.
Azmi NA, Hasham R, Ariffin FD, Elgharbawy AA, Salleh HM. Characterization, stability assessment, antioxidant evaluation and cell proliferation activityof virgin coconut oil-based nanostructured lipid carrier loaded with Ficus deltoidea extract. Cosmetics. 2020;7:1–15.
Subramaniam B, Siddik ZH, Nagoor NH. Optimization of nanostructured lipid carriers: understanding the types, designs, and parameters in the process of formulations. J Nanopart Res. 2020;22:141.
Gaba B, Fazil M, Khan S, Ali A, Baboota S, Ali J. Nanostructured lipid carrier system for topical delivery of terbinafine hydrochloride. Bull Fac Pharm Cairo Univ. 2015;53:147–59.
Haider M, Abdin SM, Kamal L, Orive G. Nanostructured lipid carriers for delivery of chemotherapeutics: a review. Pharmaceutics. 2020;12(3):288.
Sanad RA, Abdelmalak NS, Elbayoomy TS, Badawi AA. Formulation of a novel oxybenzone-loaded nanostructured lipid carriers (NLCs). AAPS PharmSci Tech. 2010;11(4):1684–94.
Taghipour B, Yakhchali M, Haririan I, Tamaddon AM, Samani SM. The effects of technical and compositional variables on the size and release profile of bovine serum albumin from PLGA based particulate systems. Res Pharm Sci. 2014;9(6):407–20.
Wang YC, Wu YT, Huang HY, Yang CS. Surfactant-free formulation of poly (lactic/glycolic) acid nanoparticles encapsulating functional polypeptide: a technical note. AAPS PharmSciTech. 2009;10(4):1263–7.
Alves DA, Machado D, Melo A, Carneiro Pereira RF, Severino P, Maria de Hollanda L, et al. Preparation of thermosensitive gel for controlled release of levofloxacin and their application in the treatment of multidrug-resistant bacteria. Biomed Res Int. 2016;2016:1–10.
Kırımlıoğlu GY, Yazan Y. Formulation and in vitro characterization of polymeric nanoparticles designed for oral delivery of levofloxacin hemihydrate. Eu Int J Sci Tech. 2016;6:655–8.
Eleraky NE, Omar MM, Mahmoud HA, Abou-Taleb HA. Nanostructured lipid carriers to mediate brain delivery of temazepam: design and in vivo study. Pharmaceutics. 2020;12(5):451.
Vestby LK, Nesse LL. Wound care antiseptics—performance differences against Staphylococcus aureus in biofilm. Acta Vet Scand. 2015;57(1):22.
Bessa LJ, Fazii P, Di Giulio M, Cellini L. Bacterial isolates from infected wounds and their antibiotic susceptibility pattern: some remarks about wound infection. Int Wound J. 2015;12(1):47–52.
Shazly GA. Ciprofloxacin controlled-solid lipid nanoparticles: characterization, in vitro release, and antibacterial activity assessment. Biomed Res Int. 2017;2120734:1–9.
Karava A, Lazaridou M, Nanaki S, Michailidou G, Christodoulou E, Kostoglou M, Iatrou H, Bikiaris DN. Chitosan derivatives with mucoadhesive and antimicrobial properties for simultaneous nanoencapsulation and extended ocular release formulations of dexamethasone and chloramphenicol drugs. Pharmaceutics. 2020;12(6):594.
Angioni E, Lercker G, Ferga NG, Carta G, et al. UV spectral properties of lipids as a tool for their identification. Eur J Lipid Sci Technol. 2002;104(1):59–64.
Shinde M, Gharge V, Pimple S, Shah M, et al. Effect of penetration enhancer on the in vitro ex vivo permeation of diclofenac gel. Asian J Pharm Clin Res. 2014:255–9.
Takeuchi H, Mano Y, Terasaka S, Sakurai T, Furuya A, Urano H, et al. Usefulness of rat skin as a substitute for human skin in the in vitro skin permeation study. Exp Anim. 2011;60(4):373–84.
Khan G, Yadav SK, Patel RR, Nath G, Bansal M, Mishra B. Development and evaluation of biodegradable chitosan films of metronidazole and levofloxacin for the management of periodontitis. AAPS Pharm SciTech. 2016;17(6):1312–25.
Acknowledgements
The authors would like to express their gratitude to the Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Helwan University for extended instrumental facility in successful accomplishment of the present work.
Author information
Authors and Affiliations
Contributions
All authors conceived and designed the experiments; Rania S. Abdel-Rashid: performed the formulations and experiment, supervision, contribution in data analysis, and writing; Eman S. El-leithy: supervision, funding, data analysis, editing; Raghda Abdel-monem: methodology, supervision, contribution in data analysis, writing and drafting manuscript to appear in its final form.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Animals experimental protocol was ethically approved by the Animal Research Ethical Committee, Faculty of Pharmacy, Helwan University.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdel-Rashid, R.S., El-leithy, E.S. & Abdel-monem, R. Formulation and Evaluation of Topical Biodegradable Films Loaded with Levofloxacin Lipid Nanocarriers. AAPS PharmSciTech 23, 34 (2022). https://doi.org/10.1208/s12249-021-02189-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02189-2