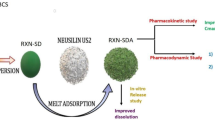
Abstract
Ticagrelor (TG) suffers from low peroral bioabsorption (36%) due to P-gp efflux and poor solubility (10 µg/mL). TG solid dispersion adsorbates (TG-SDAs) were formulated using an amalgamation of solid dispersion and melt adsorption techniques which were simple, economic, scalable, and solvent-free. FTIR indicated no incompatibility between drug and excipients. DSC, XRD, and SEM suggested a reduction in TG crystallinity. Q30min from TG-SUSP and TG-conventional tablets was only 2.30% and 6.59% respectively whereas TG-SDA-based tablets exhibited a significantly higher drug release of 86.47%. Caco-2 permeability studies showed 3.83-fold higher permeability of TG from TG-SDAs. TG-SDA-based tablets exhibited relative bioavailability of 748.53% and 153.43% compared to TG-SUSP and TG-conventional tablets respectively in rats. TG-SDA-based tablets were devoid of any cytotoxicity as indicated by MTT assay and exhibited better antiplatelet activity in rats. Enhanced oral bioavailability of TG-SDAs can be attributed to inhibition of P-gp efflux by PEG 4000, increased wettability, and reduced crystallinity of drug leading to improved drug solubility and dissolution. Improved bioabsorption results in a reduction of dose, cost of therapy as well as dose-related side effects. Thus, SDAs can be considered a promising and scalable approach for the improvement of dissolution rate and solubility of TG. TG-SDAs can be translated to an effective and safe dosage form, whereby its rapid onset of action promotes the prevention of heart attack, stroke, and related ill events in individuals with the acute coronary syndrome. However, scale-up, validation, and clinical-studies are necessary for confirmation of the proof-of-concept.












Similar content being viewed by others
References
Bajracharya R, Song JG, Back SY, et al. Recent Advancements in non-invasive formulations for protein drug delivery. Comput Struct Biotechnol J. 2019;17:1290–308.
Kalepu S, Nekkanti V. Insoluble drug delivery strategies: review of recent advances and business prospects. Acta Pharm Sin B. 2015;5:442–53.
Kumar S, Singh P. Various techniques for solubility enhancement: an overview. Pharma Innov J. 2016;5:23–8.
Arunkumar N, Deecaraman M, Rani C, et al. Preparation and solid state characterization of atorvastatin nanosuspensions for enhanced solubility and dissolution. Int J PharmTech Res. 2009;1:1725–30.
Shah H, Shah V, Bhutani S, et al. Dissolution improvement of nebivolol hydrochloride using solid dispersion adsorbate technique. Asian J Pharm. 2015;9:49–55.
Aguiar AJ, Zelmer JE. Dissolution behavior of polymorphs of chloramphenicol palmitate and mefenamic acid. J Pharm Sci. 1969;58:983–7.
Censi R, Di Martino P. Polymorph impact on the bioavailability and stability of poorly soluble drugs. Molecules. 2015;20:18759–76.
Stella VJ, Nti-Addae KW. Prodrug strategies to overcome poor water solubility. Adv Drug Deliv Rev. 2007;59:677–94.
Hamed R, Awadallah A, Sunoqrot S, et al. pH-dependent solubility and dissolution behavior of carvedilol—case example of a weakly basic BCS class II drug. AAPS PharmSciTech. 2016;17:418–26.
Boyd BJ, Bergström CAS, Vinarov Z, et al. Successful oral delivery of poorly water-soluble drugs both depends on the intraluminal behavior of drugs and of appropriate advanced drug delivery systems. Eur J Pharm Sci. 2019;137:1–27.
Sruti J, Niranjan Patra C, Swain S, et al. Improvement in the dissolution rate and tableting properties of cefuroxime axetil by melt-granulated dispersion and surface adsorption. Acta Pharm Sin B. 2013;3:113–22.
Naik NG, Sunder SR, Manchikanti Sk. A promising technique to improve the solubility by liquisolid compaction technology. J Drug Deliv Ther. 2018;8:56–61.
Afifi S. Solid dispersion approach improving dissolution rate of Stiripentol: a novel antiepileptic drug. Iran J Pharm Res. 2015;14:1001–14.
Maghsoodi M, Kiafar F. Co-precipitation with PVP and agar to improve physicomechanical properties of ibuprofen. Iran J Basic Med Sci. 2013;16:627–34.
Van NH, Park C, Oh E, et al. Improving the dissolution rate of a poorly water-soluble drug via adsorption onto pharmaceutical diluents. J Drug Deliv Sci Technol. 2016;35:146–54.
Saharan VA, Choudhury PK. Dissolution rate enhancement of gliclazide by ordered mixing. Acta Pharm. 2011;61:323–34.
Chella N, Shastri N, Tadikonda RR. Use of the liquisolid compact technique for improvement of the dissolution rate of valsartan. Acta Pharm Sin B. 2012;2:502–8.
Shanmuga Priya A, Sivakamavalli J, Vaseeharan B, et al. Improvement on dissolution rate of inclusion complex of Rifabutin drug with β-cyclodextrin. Int J Biol Macromol. 2013;62:472–80.
Saharan VA, Kukkar V, Kataria M, et al. Dissolution enhancement of drugs. Part I: Technologies and effect of carriers. Int J Health Res. 2009;2:107–24.
Kazi M. Lipid‐based nano‐delivery for oral administration of poorly water soluble drugs (PWSDs): design, optimization and in-vitro assessment. Chapter 3 from Advanced Technology for Delivering Therapeutics. Published by Intech 2017; 31–51.
Vasa DM, Dalal N, Katz JM, Roopwani R, Nevrekar A, Patel H, Buckner IS, Wildfong PLD. Physical characterization of drug:polymer dispersion behavior in polyethylene glycol 4000 solid dispersions using a suite of complementary analytical techniques. J Pharm Sci. 2014;103(9):2911–23.
Craig DQ. The mechanisms of drug release from solid dispersions in water-soluble polymers. Int J Pharm. 2002;231(2):131–44.
Serajuddin AT. Solid dispersion of poorly water-soluble drugs: early promises, subsequent problems, and recent breakthroughs. J Pharm Sci. 1999;88(10):1058–66.
Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm. 2000;50(1):47–60.
Nikam VK, Shete SK, Khapare JP. Most promising solid dispersion technique of oral dispersible tablet. Beni-Suef Univ J Basic Appl Sci. 2020;9:62.
Teng R, Oliver S, Hayes MA, et al. Absorption, distribution, metabolism, and excretion of ticagrelor in healthy subjects. Drug Metab Dispos. 2010;38:1514–21.
Na YG, Pham TMA, Byeon JJ, et al. Development and evaluation of TPGS/PVA-based nanosuspension for enhancing dissolution and oral bioavailability of ticagrelor. Int J Pharm. 2020;581:119287.
Byeon J, Son G, Jeon S, et al. Strategic approach to developing a self- microemulsifying drug delivery system to enhance antiplatelet activity and bioavailability of ticagrelor. Int J Nanomed. 2019;1193–1212.
Inam M, Wu J, Shen J, et al. Preparation and characterization of novel pharmaceutical co-crystals: ticagrelor with nicotinamide. Curr Comput-Aided Drug Des. 2018;8(9):336.
Bayoumi AA. Enhancement of solubility of a poorly soluble antiplatelet aggregation drug by cogrinding technique. Asian J Pharm Clin Res. 2018;11(10):340–4.
Kim SJ, Lee HK, Na YG, et al. A novel composition of ticagrelor by solid dispersion technique for increasing solubility and intestinal permeability. Int J Pharm. 2019;555:11–8.
Kim Y, Chen J. Molecular structure of human P-glycoprotein in the ATP-bound, outward-facing conformation. Science. 2018;359:915–9.
Dua K, Pabreja K, Ramana MV, et al. Preparation, characterization, and in-vitro evaluation of aceclofenac PVP-solid dispersions. J Dispers Sci Technol. 2011;32:1151–7.
Dua K, Pabreja K, Ramana MV. Enhancement of dissolution behavior of aceclofenac by complexation with β-cyclodextrin-choline dichloride coprecipitate. J Dispers Sci Technol. 2011;32:1477–84.
Lyn L, Sze H, Rajendran A, et al. Crystal modifications and dissolution rate of piroxicam. Acta Pharm. 2011;61:391–402.
Gupta MK, Tseng YC, Goldman D, et al. Hydrogen bonding with adsorbent during storage governs drug dissolution from solid-dispersion granules. Pharm Res. 2002;19:1663–72.
Mahajan A, Surti N, Koladiya P. Solid dispersion adsorbate technique for improved dissolution and flow properties of lurasidone hydrochloride: characterization using 32 factorial design. Drug Dev Ind Pharm. 2018;44:463–71.
Vojinović T, Medarević D, Vranić E, et al. Development of ternary solid dispersions with hydrophilic polymer and surface adsorbent for improving dissolution rate of carbamazepine. Saudi Pharm J. 2018;26:725–32.
Kumria R, Al-Dhubiab BE, Shah J, et al. Formulation and evaluation of chitosan-based buccal bioadhesive films of zolmitriptan. J Pharm Innov. 2018;13:133–43.
Takeichi Y, Shimooka T, Yamabe K, et al. Improvement of solubility and oral bioavailability of a poorly water-soluble drug, TAS-301, by its melt-adsorption on a porous calcium silicate. J Pharm Sci. 2002;91:362–70.
Jacob S, Shirwaikar A, Nair A. Preparation and evaluation of fast-disintegrating effervescent tablets of glibenclamide. Drug Dev Ind Pharm. 2009;35:321–8.
Shah RB, Tawakkul MA, Khan MA. Comparative evaluation of flow for pharmaceutical powders and granules. AAPS PharmSciTech. 2008;9:250–8.
Govedarica B, Injac R, Dreu R, et al. Formulation and evaluation of immediate release tablets with different types of paracetamol powders prepared by direct Compression. African J Pharm Pharmacol. 2011;5:31–41.
US Pharmacopeia. Tablets Compendial tests. United States Pharmacop. 2010;5637–5640.
Saker A, Cares-Pacheco MG, Marchal P, et al. Powders flowability assessment in granular compaction: what about the consistency of Hausner ratio? Powder Technol. 2019;354:52–63.
Ganesan V, Rosentrater KA, Muthukumarappan K. Flowability and handling characteristics of bulk solids and powders - a review with implications for DDGS. Biosyst Eng. 2008;101:425–35.
Kumar C, Kumar M, Saini V, et al. Dissolution method development and validation for combination dosage form of telmisartan and nebivolol hydrochloride tablets using UV spectrophotometric method. Res J Pharm Technol. 2019;12:2742–7.
Rajesh A, Manmeet K, Sangeeta A. Formulation and evaluation of fast dissolving tablet of pioglitazone. Res J Pharm Technol. 2012;5:817–21.
Shah P, Sarolia J, Vyas B, Wagh P, Kaul A, Mishra AK. PLGA Nanoparticles for nose to brain delivery of clonazepam: formulation, optimization by 32 factorial design, in-vitro and in-vivo evaluation. Curr Drug Deliv. 2020. https://doi.org/10.2174/1567201817666200708115627.
Borderwala K, Swain G, Mange N, Gandhi J, Lalan M, Singhvi G, Shah P. Optimization of solid lipid nanoparticles of ezetimibe in combination with simvastatin using quality by design (QbD). Nanosci Nanotechnol Asia. 2020;10(4):404–18.
Naik B, Gandhi J, Shah P, Naik H, Sarolia J. Asenapine Maleate Loaded Solid Lipid Nanoparticles for Oral Delivery. Int Res J Pharm. 2017;8:45–53.
Pathak B, Raghav M, Thakkar A, Vyas B, Shah P. Enhanced oral bioavailability of etodolac by the liquisolid compact technique: optimisation, in-vitro and in-vivo evaluation. Curr Drug Deliv. 2021;18(4):471–86.
Nair AB, Al-Dhubiab BE, Shah J, et al. Development and evaluation of palonosetron loaded mucoadhesive buccal films. J Drug Deliv Sci Technol. 2018;47:351–8.
Shah H, Nair AB, Shah J, et al. Proniosomal gel for transdermal delivery of lornoxicam: optimization using factorial design and in-vivo evaluation in rats. Daru. 2019;27:59–70.
Zhao YM, Zheng ZB, Li S. Quantification of flupirtine maleate polymorphs using X-ray powder diffraction. Chinese Chem Lett. 2016;27:1666–72.
Pilli R, Pradesh A. Etodolac dissolution improvement by preparation of solid dispersions. Int J Pharm Sci Res. 2014;5:4774–91.
Mohamed JMM, Alqahtani A, Ahmad F, Krishnaraju V, Kalpana K. Stoichiometrically governed curcumin solid dispersion and its cytotoxic evaluation on colorectal adenocarcinoma cells. Drug Des Devel Ther. 2020;14:4639–58.
Choi JS, Cho NH, Kim DH, et al. Comparison of paclitaxel solid dispersion and polymeric micelles for improved oral bioavailability and in-vitro anti-cancer effects. Mater Sci Eng C. 2019;100:247–59.
Shah P, Chavda K, Vyas B, Patel S. Formulation development of linagliptin solid lipid nanoparticles for oral bioavailability enhancement: role of P-gp inhibition. Drug Deliv Transl Res. 2021;11(3):1166–85.
Chang CW, Wong CY, Wu YT, et al. Development of a solid dispersion system for improving the oral bioavailability of resveratrol in rats. Eur J Drug Metab Pharmacokinet. 2017;42:239–49.
Sugidachi A, Asai F, Ogawa T, et al. The in-vivo pharmacological profile of CS-747, a novel antiplatelet agent with platelet ADP receptor antagonist properties. Br J Pharmacol. 2000;129:1439–46.
Wang YY, Tang ZY, Dong M, et al. Inhibition of platelet aggregation by polyaspartoyl L-arginine and its mechanism. Acta Pharmacol Sin. 2004;25:469–73.
Mekhfi H, El HM, Legssyer A, et al. Platelet anti-aggregant property of some Moroccan medicinal plants. J Ethnopharmacol. 2004;94:317–22.
Brown EW. © 1962 Nature Publishing Group. Nat Int J Sci. 1962;196:1048–50.
Narang AS, Srivastava AK. Evaluation of solid dispersions of Clofazimine. Drug Dev Ind Pharm. 2002;28(8):1001–13.
Lavra ZMM, Pereira de Santana D, Ré MI. Solubility and dissolution performances of spray-dried solid dispersion of Efavirenz in Soluplus. Drug Dev Ind Pharm. 2017;43:42–54.
Lee HJ, Kim JY, Park SH, et al. Controlled-release oral dosage forms containing nimodipine solid dispersion and hydrophilic carriers. J Drug Deliv Sci Technol. 2017;37:28–37.
Taokaew S, Ofuchi M, Kobayashi T. Chitin biomass-nifedipine amorphous solid dispersion for enhancement of hydrophobic drug dissolution in aqueous media. Sustain Chem Pharm. 2020;17:100284.
Jo K, Cho JM, Lee H, et al. Enhancement of aqueous solubility and dissolution of celecoxib through phosphatidylcholine-based dispersion systems solidified with adsorbent carriers. Pharmaceutics. 2019;11:1–14.
Hoppe K, Sznitowska M. The effect of polysorbate 20 on solubility and stability of candesartan cilexetil in dissolution media. AAPS PharmSciTech. 2014;15:1116–25.
Gupta MK, Goldman D, Bogner RH, et al. Enhanced drug dissolution and bulk properties of solid dispersions granulated with a surface adsorbent. Pharm Dev Technol. 2001;6:563–72.
Kapsi SG, Ayres JW. Processing factors in development of solid solution formulation of itraconazole for enhancement of drug dissolution and bioavailability. Int J Pharm. 2001;229:193–203.
Chutimaworapan S, Ritthidej GC, Yonemochi E, et al. Effect of water-soluble carriers on dissolution characteristics of nifedipine solid dispersions. Drug Dev Ind Pharm. 2000;26:1141–50.
Wen T, Niu B, Wu Q, et al. Fenofibrate solid dispersion processed by hot-melt extrusion: elevated bioavailability and its cell transport mechanism. Curr Drug Deliv. 2019;16:538–47.
Dobesh PP, Oestreich JH. Ticagrelor: pharmacokinetics, pharmacodynamics, clinical efficacy, and safety. Pharmacotherapy. 2014;34(10):1077–90.
Nylander S, Femia EA, Scavone M, Berntsson P, Asztély AK, Nelander K, Cattaneo M. Ticagrelor inhibits human platelet aggregation via adenosine in addition to P2Y12 antagonism. J Thromb Haemost. 2013;11(10):1867–76.
Fule R, Amin P. Development and evaluation of lafutidine solid dispersion via hot melt extrusion: Investigating drug-polymer miscibility with advanced characterisation. Asian J Pharm Sci. 2014;9(2):92–106.
Funding
This study is funded by the Maliba Pharmacy College.
Author information
Authors and Affiliations
Contributions
Mukesh Yadav: data collection; Jayant Sarolia: data collection; Manisha Lalan: draft manuscript preparation; Shubhada Mangrulkar Bhavin Vyas: analysis and interpretation of results; Bhavin Vyas: study conception and design; Pranav Shah: study conception and design. All the authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yadav, M., Sarolia, J., Vyas, B. et al. Amalgamation of Solid Dispersion and Melt Adsorption Technique: Improved In Vitro and In Vivo Performance of Ticagrelor Tablets. AAPS PharmSciTech 22, 257 (2021). https://doi.org/10.1208/s12249-021-02138-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02138-z