Abstract
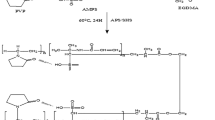
In this study, two hydrophilic polymers hydroxypropyl methyl cellulose and beta-cyclodextrin (β-CD) are used to synthesize highly responsive and spongy polymeric matrices. Porous and stimulus-responsive polymeric network was developed to improve the solubility of acyclovir (ACV) at significant level. Grafting was successfully carried out by free radical polymerization technique. Spongy matrices were characterized by percentage entrapment efficiency, drug loading, solubility studies, FTIR, powder X-ray diffraction, TGA, DSC, XRD, SEM, swelling studies, and in vitro studies. Acute oral toxicity studies were conducted to determine the safety of oral administration of prepared HPMC-βCD-g-poly(AMPS) formulation. Porous and spongy structures were depicted in SEM images. Complex formation and thermal stability of constituents and drug (ACV) were analyzed by FTIR, TGA, and DSC spectra. XRD analysis revealed reduction in acyclovir crystallinity in spongy matrices. Particle size of optimized formulation was found in the range of 197 ± 2.55 nm. The momentous difference with reference product committed that drug solubility and release characteristics were markedly enhanced by the developed spongy matrices. Toxicity studies endorsed that developed spongy matrices were non-toxic and compatible to biological system. The efficient method of preparation, enhanced solubility, excellent physico-chemical characteristics, high dissolution, and non-toxic HPMC-βCD-g-poly(AMPS) spongy matrices may be a promising approach for oral delivery of poorly soluble drugs.











Similar content being viewed by others
References
Mahmood A, Ahmad M, Sarfraz RM, Minhas MU. β-CD based hydrogel microparticulate system to improve the solubility of acyclovir: optimization through in-vitro, in-vivo and toxicological evaluation. J Drug Deliv Sci Technol. 2016;36:75–88.
Mahmood A, Ahmad M, Sarfraz RM, Usman Minhas M. Development of acyclovir loaded β-cyclodextrin-g-poly methacrylic acid hydrogel microparticles: an in vitro characterization. Adv Polym Technol. 2018;37(3):697–705.
Celebioglu A, Uyar T. Electrospun formulation of acyclovir/cyclodextrin nanofibers for fast-dissolving antiviral drug delivery. Mater Sci Eng C. 2021;118:111514.
Bhandare R, Londhe V, Ashames A, Shaikh N, Alabdin SZ. Enhanced solubility of microwave-assisted synthesized acyclovir Co-crystals. Res J Pharm Tech. 2020;13:5979–86.
Jermain SV, Brough C, Williams RO III. Amorphous solid dispersions and nanocrystal technologies for poorly water-soluble drug delivery–an update. Int J Pharm. 2018;535(1-2):379–92.
Tambe A, Pandita N. Enhanced solubility and drug release profile of boswellic acid using a poloxamer-based solid dispersion technique. J Drug Deliv Sci Technol. 2018;44:172–80.
Khan KU, et al. Synthesis of PEG-4000-co-poly (AMPS) nanogels by cross-linking polymerization as highly responsive networks for enhancement in meloxicam solubility. Drug Dev Ind Pharm. 2021:1–12.
Hecq J, Deleers M, Fanara D, Vranckx H, Amighi K. Preparation and characterization of nanocrystals for solubility and dissolution rate enhancement of nifedipine. Int J Pharm. 2005;299(1-2):167–77.
Dong B, Hadinoto K. Amorphous nanoparticle complex of perphenazine and dextran sulfate as a new solubility enhancement strategy of antipsychotic perphenazine. Drug Dev Ind Pharm. 2017;43(6):996–1002.
Naqvi A, et al. Preparation and evaluation of pharmaceutical co-crystals for solubility enhancement of atorvastatin calcium. Polym Bull. 2019.
Yan T, Ji M, Sun Y, Yan T, Zhao J, Zhang H, et al. Preparation and characterization of baicalein/hydroxypropyl-β-cyclodextrin inclusion complex for enhancement of solubility, antioxidant activity and antibacterial activity using supercritical antisolvent technology. J Incl Phenom Macrocycl Chem. 2020;96(3):285–95.
Morina D, Sessevmez M, Sinani G, Mülazımoğlu L, Cevher E. Oral tablet formulations containing cyclodextrin complexes of poorly water soluble cefdinir to enhance its bioavailability. J Drug Deliv Sci Technol. 2020;57:101742.
Amidi M, Mastrobattista E, Jiskoot W, Hennink WE. Chitosan-based delivery systems for protein therapeutics and antigens. Adv Drug Deliv Rev. 2010;62(1):59–82.
Hua D, Jiang J, Kuang L, Jiang J, Zheng W, Liang H. Smart chitosan-based stimuli-responsive nanocarriers for the controlled delivery of hydrophobic pharmaceuticals. Macromolecules. 2011;44(6):1298–302.
Kayaman-Apohan N, Akyürek E. Synthesis and drug-release properties of biodegradable hydrogels having β-cyclodextrin. Polym Bull. 2013;70(8):2151–67.
Loftsson T, Brewster ME, Masson M. Role of cyclodextrins in improving oral drug delivery. Am J Drug Deliv. 2004;2(4):261–75.
Bilensoy E, Hincal AA. Recent advances and future directions in amphiphilic cyclodextrin nanoparticles. Expert Opin Drug Deliv. 2009;6(11):1161–73.
Trotta F, Dianzani C, Caldera F, Mognetti B, Cavalli R. The application of nanosponges to cancer drug delivery. Expert Opin Drug Deliv. 2014;11(6):931–41.
Giulbudagian M, Hönzke S, Bergueiro J, Işık D, Schumacher F, Saeidpour S, et al. Enhanced topical delivery of dexamethasone by β-cyclodextrin decorated thermoresponsive nanogels. Nanoscale. 2018;10(1):469–79.
Kendre P, Satav T. Current trends and concepts in the design and development of nanogel carrier systems. Polym Bull. 2019;76(3):1595–617.
Ali L, Ahmad M, Usman M. Evaluation of cross-linked hydroxypropyl methylcellulose graft-methacrylic acid copolymer as extended release oral drug carrier. Cellul Chem Technol. 2015;49(2):143–51.
Siepmann J, Peppas NaA. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv Drug Deliv Rev. 2012;64:163–74.
Hazer O, Soykan C, Kartal Ş. Synthesis and swelling behavior analysis of poly(acrylamidoxime-co-2-acrylamido-2-methylpropane sulfonic acid) hydrogels. Journal of Macromolecular Science, Part A: Pure and Applied Chemistry. 2007;45(1):45–51.
Kim SJ, Lee CK, Kim SI. Electrical/pH responsive properties of poly (2-acrylamido-2-methylpropane sulfonic acid)/hyaluronic acid hydrogels. J Appl Polym Sci. 2004;92(3):1731–6.
Pourjavadi A, Barzegar S, Zeidabadi F. Synthesis and properties of biodegradable hydrogels of κ-carrageenan grafted acrylic acid-co-2-acrylamido-2-methylpropanesulfonic acid as candidates for drug delivery systems. React Funct Polym. 2007;67(7):644–54.
Khan KU, Minhas MU, Sohail M, Badshah SF, Abdullah O, Khan S, et al. Synthesis of PEG-4000-co-poly (AMPS) nanogels by cross-linking polymerization as highly responsive networks for enhancement in meloxicam solubility. Drug Dev Ind Pharm. 2021;47(3):465–76.
Rao KM, et al. Novel thermo/pH sensitive nanogels composed from poly (N-vinylcaprolactam) for controlled release of an anticancer drug. Colloids Surf B: Biointerfaces. 2013;102:891–7.
Bode C, Kranz H, Fivez A, Siepmann F, Siepmann J. Often neglected: PLGA/PLA swelling orchestrates drug release: HME implants. J Control Release. 2019;306:97–107.
Jain S, Ancheria RK, Shrivastava S, Soni SL, Sharma M. An overview of nanogel–novel drug delivery system. Asian Journal of Pharmaceutical Research and Development. 2019;7(2):47–55.
Minhas MU, et al. Synthesis and characterization of β-cyclodextrin hydrogels: crosslinked polymeric network for targeted delivery of 5-fluorouracil. Drug Deliv. 2016;9:10.
Taleb MA, Hegazy DE, Mahmoud GA. Characterization and in vitro drug release behavior of (2-hydroxyethyl methacrylate)–co-(2-acrylamido-2-methyl-1-propanesulfonic acid) crosslinked hydrogels prepared by ionizing radiation. Int J Polym Mater Polym Biomater. 2014;63(16):840–5.
Suhail M, Khan A, Rosenholm JM, Minhas MU, Wu PC. Fabrication and characterization of diclofenac sodium loaded hydrogels of sodium alginate as sustained release carrier. Gels. 2021;7(1):10.
Wang W, Wang A. Synthesis and swelling properties of pH-sensitive semi-IPN superabsorbent hydrogels based on sodium alginate-g-poly (sodium acrylate) and polyvinylpyrrolidone. Carbohydr Polym. 2010;80(4):1028–36.
Minhas MU, et al. Functionalized pectin hydrogels by cross-linking with monomer: synthesis, characterization, drug release and pectinase degradation studies. Polym Bull. 2020;77(1):339–56.
Khalid Q, Ahmad M, Minhas MU. Synthesis of β-cyclodextrin hydrogel nanoparticles for improving the solubility of dexibuprofen: characterization and toxicity evaluation. Drug Dev Ind Pharm. 2017;43(11):1873–84.
Chen Y, Liu Y. Cyclodextrin-based bioactive supramolecular assemblies. Chem Soc Rev. 2010;39(2):495–505.
Sadeghi M, Hosseinzadeh H. Synthesis and super-swelling behavior of a novel low salt-sensitive protein-based superabsorbent hydrogel: collagen-g-poly (AMPS). Turk J Chem. 2010;34(5):739–52.
Khalid Q, Ahmad M, Usman Minhas M. Hydroxypropyl-β-cyclodextrin hybrid nanogels as nano-drug delivery carriers to enhance the solubility of dexibuprofen: Characterization, in vitro release, and acute oral toxicity studies. Adv Polym Technol. 2018;37(6):2171–85.
Mutar MA, Radia ND. Controlled release from crosslinked polyacrylic acid as drug delivery theophylline. Irq Nat J Chem. 2012;45:67–85.
Khan KU, Akhtar N, Minhas MU. Poloxamer-407-co-poly (2-acrylamido-2-methylpropane sulfonic acid) cross-linked nanogels for solubility enhancement of olanzapine: synthesis, characterization, and toxicity evaluation. AAPS PharmSciTech. 2020;21:1–15.
Guo C, et al. Optimization of extended-release ZL-004 nanosuspensions for in vivo pharmacokinetic study to enhance low solubility and compliance. Molecules. 2019;24(1):7.
Ren X, Qi J, Wu W, Yin Z, Li T, Lu Y. Development of carrier-free nanocrystals of poorly water-soluble drugs by exploring metastable zone of nucleation. Acta Pharm Sin B. 2019;9(1):118–27.
Farooq U, et al. Development, characterization and evaluation of anti-fungal activity of miconazole based nanogel prepared from biodegradable polymer. Pak J Pharm Sci. 2020;33(1 (Special)):449.
Badshah SF, Akhtar N, Minhas MU, Khan KU, Khan S, Abdullah O, et al. Porous and highly responsive cross-linked β-cyclodextrin based nanomatrices for improvement in drug dissolution and absorption. Life Sci. 2021;267:118931.
Ranjha NM, Qureshi UF. Preparation and characterization of crosslinked acrylic acid/hydroxypropyl methyl cellulose hydrogels for drug delivery. Int J Pharm Pharm Sci. 2014;6(400):410.
Clara I, Lavanya R, Natchimuthu N. pH and temperature responsive hydrogels of poly (2-acrylamido-2-methyl-1-propanesulfonic acid-co-methacrylic acid): synthesis and swelling characteristics. J Macromol Sci A. 2016;53(8):492–9.
Wang Y, et al. Synthesis, characterization, and swelling behaviors of a pH-responsive CMC-g-poly (AA-co-AMPS) superabsorbent hydrogel. Turk J Chem. 2013;37(1):149–59.
Sohail K, Khan IU, Shahzad Y, Hussain T, Ranjha NM. pH-sensitive polyvinylpyrrolidone-acrylic acid hydrogels: Impact of material parameters on swelling and drug release. Braz J Pharm Sci. 2014;50(1):173–84.
Ghorpade VS, Yadav AV, Dias RJ. Citric acid crosslinked cyclodextrin/hydroxypropylmethylcellulose hydrogel films for hydrophobic drug delivery. Int J Biol Macromol. 2016;93:75–86.
Rastogi PK, Krishnamoorthi S, Ganesan V. Synthesis, characterization, and ion exchange voltammetry study on 2-acrylamido-2-methylpropane sulphonic acid and N-(hydroxymethyl) acrylamide-based copolymer. J Appl Polym Sci. 2012;123(2):929–35.
Anwar H, Ahmad M, Minhas MU, Rehmani S. Alginate-polyvinyl alcohol based interpenetrating polymer network for prolonged drug therapy, optimization and in-vitro characterization. Carbohydr Polym. 2017;166:183–94.
Malik NS, Ahmad M, Minhas MU. Cross-linked β-cyclodextrin and carboxymethyl cellulose hydrogels for controlled drug delivery of acyclovir. PLoS One. 2017;12(2):e0172727.
Sarfraz RM, et al. Development of β-cyclodextrin-based hydrogel microparticles for solubility enhancement of rosuvastatin: an in vitro and in vivo evaluation. Drug Des Devel Ther. 2017;11:3083.
Dharmalingam K, Anandalakshmi R. Fabrication, characterization and drug loading efficiency of citric acid crosslinked NaCMC-HPMC hydrogel films for wound healing drug delivery applications. Int J Biol Macromol. 2019;134:815–29.
Kabiri K, et al. Super-alcogels based on 2-acrylamido-2-methylpropane sulphonic acid and poly (ethylene glycol) macromer; 2011.
Ford JL. Thermal analysis of hydroxypropylmethylcellulose and methylcellulose: powders, gels and matrix tablets. Int J Pharm. 1999;179(2):209–28.
Khanum H, Ullah K, Murtaza G, Khan SA. Fabrication and in vitro characterization of HPMC-g-poly (AMPS) hydrogels loaded with loxoprofen sodium. Int J Biol Macromol. 2018;120:1624–31.
Zhang C, Easteal AJ. Study of free-radical copolymerization of N-isopropylacrylamide with 2-acrylamido-2-methyl-1-propanesulphonic acid. J Appl Polym Sci. 2003;88(11):2563–9.
Cheng W-M, Hu XM, Wang DM, Liu GH. Preparation and characteristics of corn straw-Co-AMPS-Co-AA superabsorbent hydrogel. Polymers. 2015;7(11):2431–45.
Rao M, Bajaj A, Khole I, Munjapara G, Trotta F. In vitro and in vivo evaluation of β-cyclodextrin-based nanosponges of telmisartan. J Incl Phenom Macrocycl Chem. 2013;77(1-4):135–45.
Guo Y, Shalaev E, Smith S. Physical stability of pharmaceutical formulations: solid-state characterization of amorphous dispersions. TrAC Trends Anal Chem. 2013;49:137–44.
Acknowledgements
This research was supported by the Faculty of Pharmacy, the Islamia University of Bahawalpur, Punjab, Pakistan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 192 kb)
Rights and permissions
About this article
Cite this article
Asghar, S., Akhtar, N., Minhas, M.U. et al. Bi-polymeric Spongy Matrices Through Cross-linking Polymerization: Synthesized and Evaluated for Solubility Enhancement of Acyclovir. AAPS PharmSciTech 22, 181 (2021). https://doi.org/10.1208/s12249-021-02054-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02054-2