Abstract
This research aimed to develop a novel drug delivery system to improve treatment of skin disorders. The system is comprised of a Carbopol 980-based nanoemulgel (NE-gel) containing a desonide (DES; 0.05%, w/w) nanoemulsion (NE), which has a small particle size, high encapsulation efficiency, good thermodynamic stability, good permeation ability, and high skin retention. DES-loaded NE (DES-NE) was prepared by high-pressure homogenization. The developed formulation was characterized by differential scanning calorimetry (DSC), X-ray diffraction, drug release, skin permeation, and drug retention. DES in vitro release and skin permeation studies with different formulations of artificial membrane and rat abdominal skin were performed with the Franz diffusion cell system. Confocal laser scanning microscopy (CLSM) was used to detect the localization and permeation pathways of drugs in the skin. Compared with commercially available gel (CA-gel) and NE, the NE-gel release process conformed to the Higuchi release model (R2 = 0.9813). NE-gel prolonged the drug release time and allowed for reduced administration dose and frequency. The unit cumulative permeation of NE and NE-gel through the skin for 12 h was 63.13 ± 2.78 and 42.53 ± 2.06 μg/cm2, respectively, values significantly higher (p < 0.01) than that of the CA-gel (30.65 ± 1.25 μg/cm2) and CA-cream (15.21 ± 0.97 μg/cm2). The DES-NE and DES NE-gel skin drug retention was significantly higher than commercially available formulations (p < 0.01). Hence, the prepared NE-gel is a potential vehicle for improved topical DES delivery for better treatment of skin disorders.












Similar content being viewed by others
References
Ali MS, Alam MS, Alam N, Siddiqui MR. Preparation, characterization and stability study of dutasteride loaded nanoemulsion for treatment of benign prostatic hypertrophy. Iran J Pharm Res. 2014;13(4):1125–40.
Gelbard CM, Hebert AA. Desonide hydrogel: advances in vehicle technology. Expert Rev Dermatol. Taylor & Francis. 2009;4:23–7.
Pradhan M, Singh D, Singh MR. Influence of selected variables on fabrication of Triamcinolone acetonide loaded solid lipid nanoparticles for topical treatment of dermal disorders. Artif Cells Nanomed Biotechnol. 2016;44(1):392–400.
Antonow MB, Lorenzoni R, Barbosa GM, Ourique AF, Gomes P, Raffin RP. Development and physicochemical characterization of desonide-loaded nanocapsule suspensions. Adv Mater Sci Eng. 2016;2016:1–12.
Bamrungsap S, Zhao Z, Chen T, Wang L, Li C, Fu T, et al. Nanotechnology in therapeutics: A focus on nanoparticles as a drug delivery system. Nanomedicine. 2012;7(8):1253–71.
Mahtab A, Anwar M, Mallick N, Naz Z, Jain GK, Ahmad FJ. Transungual delivery of ketoconazole nanoemulgel for the effective management of onychomycosis. AAPS PharmSciTech. 2016;17(6):1477–90.
Jaiswal M, Dudhe R, Sharma PK. Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech. 2015;5(2):123–7.
Kaur A, Katiyar SS, Kushwah V, Jain S. Nanoemulsion loaded gel for topical co-delivery of clobitasol propionate and calcipotriol in psoriasis. Nanomedicine. 2017;13(4):1473–82.
Somagoni J, Boakye CHA, Godugu C, Patel AR, Faria HAM, Zucolotto V, et al. Nanomiemgel - A novel drug delivery system for topical application - In vitro and in vivo evaluation. PLoS One. 2014;e115952.
Sutradhar KB, Amin L. Nanoemulsions: Increasing possibilities in drug delivery. Eur J Nanomed. 2013;5(2):97–110.
Shaker DS, Ishak RAH, Ghoneim A, Elhuoni MA. Nanoemulsion: a review on mechanisms for the transdermal delivery of hydrophobic and hydrophilic drugs. Sci Pharm. 2019;87(3):17.
Chellapa P, Mohamed AT, Keleb EI, Elmahgoubi A, Eid AM, Issa YS, et al. Nanoemulsion and nanoemulgel as a topical formulation. IOSR J Pharm. 2015;5:43–7.
Sharma A, Singh AP, Harikumar SL. Development and optimization of nanoemulsion based gel for enhanced transdermal delivery of nitrendipine using box-behnken statistical design. Drug Dev Ind Pharm. 2020;46(2):329–42.
Krahn CL, Raffin RP, Santos GS, Queiroga LB, Cavalcanti RL, Serpa P, et al. Isoflurane-loaded nanoemulsion prepared by high-pressure homogenization: Investigation of stability and dose reduction in general anesthesia. J Biomed Nanotechnol. 2012;8(5):849–58.
Belletti D, Vandelli MA, Tonelli M, Zapparoli M, Forni F, Tosi G, et al. Functionalization of liposomes: microscopical methods for preformulative screening. J Liposome Res. 2015;25(2):150–6.
Azeem A, Rizwan M, Ahmad FJ, Iqbal Z, Khar RK, Aqil M, et al. Nanoemulsion components screening and selection: a technical note. AAPS PharmSciTech. 2009;10(1):69–76.
Dasgupta S, Dey S, Choudhury S, Mazumder B. Topical delivery of aceclofenac as nanoemulsion comprising excipients having optimum emulsification capabilities: preparation, characterization and in vivo evaluation. Exp Opin Drug Deliv. 2013;10(4):411–20.
Ahmad J, Gautam A, Komath S, Bano M, Garg A, Jain K. Topical nano-emulgel for skin disorders: formulation approach and characterization. Recent Pat Antiinfect Drug Discov. 2019;14(1):36–48.
Modi JD, Patel JK. Nanoemulsion-based gel formulation of aceclofenac for topical delivery. Int J. 2011;1(1):6–12.
Kaur R, Ajitha M. Formulation of transdermal nanoemulsion gel drug delivery system of lovastatin and its in vivo characterization in glucocorticoid induced osteoporosis rat model. J Drug Deliv Sci Technol. 2019;52:968–78.
Jangdey MS, Gupta A, Saraf S. Fabrication, in-vitro characterization, and enhanced in-vivo evaluation of carbopol-based nanoemulsion gel of apigenin for uv-induced skin carcinoma. Drug Deliv. 2017;24(1):1026–36.
Garg BJ, Garg NK, Beg S, Singh B, Katare OP. Nanosized ethosomes-based hydrogel formulations of methoxsalen for enhanced topical delivery against vitiligo: Formulation optimization, in vitro evaluation and preclinical assessment. J Drug Target. 2016;24(3):233–46.
Mohammed MI, Makky AMA, Teaima MHM, Abdellatif MM, Hamzawy MA, Khalil MAF. Transdermal delivery of vancomycin hydrochloride using combination of nano-ethosomes and iontophoresis: in vitro and in vivo study. Drug Deliv. 2016;23(5):1558–64.
Mou D, Chen H, Du D, Mao C, Wan J, Xu H, et al. Hydrogel-thickened nanoemulsion system for topical delivery of lipophilic drugs. Int J Pharm. 2008;353(1-2):270–6.
Abdellatif MM, Khalil IA, Khalil MAF. Sertaconazole nitrate loaded nanovesicular systems for targeting skin fungal infection: In-vitro, ex-vivo and in-vivo evaluation. Int J Pharm. 2017;527(1-2):1–11.
Radhika PR, Guruprasad S. Nanoemulsion based emulgel formulation of lipophilic drug for topical delivery. Int J PharmTech Res. 2016;9(6):210–23.
Levy MY, Schutze W, Fuhrer C, Benita S. Characterization of diazepam submicron emulsion interface: role of oleic acid. J Microencapsul. 1994;11(1):79–92.
Osborne DW. Diethylene glycol monoethyl ether: an emerging solvent in topical dermatology products. J Cosmet Dermatol. 2011;10(4):324–9.
Barakat N. Formulation design of indomethacin-loaded nanoemulsion for transdermal delivery. Pharm Anal Acta. 2011;2:1–8.
Javadzadeh Y, Adibkia K, Hamishekar H. Transcutol® (diethylene glycol monoethyl ether): a potential penetration enhancer. In: Dragicevic N, Maibach H, editors. Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement. Berlin: Springer; 2015. p. 195–205.
Bialik W, Walters KA, Brain KR, Hadgraft J. Some factors affecting the in vitro penetration of ibuprofen through human skin. Int J Pharm. 1993;92(1-3):219–23.
Alam M, Sajid M, Alam N, Alam M, Anwer T, Imam F, et al. Design and characterization of nanostructure topical gel of betamethasone dipropionate for psoriasis. J Appl Pharm Sci. 2012;2:148–58.
Herman A, Herman AP. Essential oils and their constituents as skin penetration enhancer for transdermal drug delivery: a review. J Pharm Pharmacol. 2015;67(4):473–85.
Sivakumar M, Tang SY, Tan KW. Cavitation technology - a greener processing technique for the generation of pharmaceutical nanoemulsions. Ultrason Sonochem. 2014.
Schmidts T, Dobler D, Nissing C, Runkel F. Influence of hydrophilic surfactants on the properties of multiple W/O/W emulsions. J Colloid Interface Sci. 2009;338(1):184–92.
Mahdi ES, Sakeena MH, Abdulkarim MF, Abdullah GZ, Sattar MA, Noor AM. Effect of surfactant and surfactant blends on pseudoternary phase diagram behavior of newly synthesized palm kernel oil esters. Drug Des Devel Ther. 2011;5:311.
Capek I. Degradation of kinetically-stable o/w emulsions. Adv Colloid Interf Sci. 2004;107(2-3):125–55.
Baspinar Y, Keck CM, Borchert HH. Development of a positively charged prednicarbate nanoemulsion. Int J Pharm. 2010;383(1-2):201–8.
Junghanns JU, Buttle I, Müller RH, Araújo IB, Silva AKA, Egito EST, et al. SolEmuls® technology: A way to overcome the drawback of parenteral administration of insoluble drugs. Pharm Dev Technol. 2008;12(5):437–45.
Maia CS, Mehnert W, Schaller M, Korting HC, Gysler A, Haberland A, et al. Drug targeting by solid lipid nanoparticles for dermal use. J Drug Target. 2002;10(6):489–95.
Schoellhammer CM, Blankschtein D, Langer R. Skin permeabilization for transdermal drug delivery: Recent advances and future prospects. Expert Opin Drug Deliv. 2014;11(3):393–407.
Ding Z, Jiang Y, Liu X. Nanoemulsios-based drug delivery for brain tumors. Nanotechnol-Based Target Drug Deliv Syst Brain Tumors. 2018:327–58.
Ahmad N, Ahmad R, Al-Qudaihi A, Alaseel SE, Fita IZ, Khalid MS, et al. Preparation of a novel curcumin nanoemulsion by ultrasonication and its comparative effects in wound healing and the treatment of inflammation. RSC Adv. 2019;9(35):20192–206.
Oh DH, Kang JH, Kim DW, Lee BJ, Kim JO, Yong CS, et al. Comparison of solid self-microemulsifying drug delivery system (solid SMEDDS) prepared with hydrophilic and hydrophobic solid carrier. Int J Pharm. 2011;420(2):412–8.
Ahmad J, Mir SR, Kohli K, Amin S. Effect of oil and co-surfactant on the formation of Solutol HS 15 based colloidal drug carrier by Box-Behnken statistical design. Colloids Surf A Physicochem Eng Asp. 2014;453:68–77.
Ouyang L, Wu Z, Wang J, Qi X, Li Q, Wang J, et al. The effect of solid content on the rheological properties and microstructures of a Li-ion battery cathode slurry. RSC Adv. 2020;10(33):19360–70.
Xue D, Sethi R. Viscoelastic gels of guar and xanthan gum mixtures provide long-term stabilization of iron micro- and nanoparticles. J Nanopart Res. 2012;14(11):1–14.
Sengupta P, Chatterjee B. Potential and future scope of nanoemulgel formulation for topical delivery of lipophilic drugs. Int J Pharm. Elsevier B.V. 2017;526:353–65.
Arora R, Aggarwal G, Harikumar SL, Kaur K. Nanoemulsion based hydrogel for enhanced transdermal delivery of ketoprofen. Adv Pharm. 2014;2014.
Frelichowska J, Bolzinger M, Pelletier J, Valour J, Chevalier Y. Topical delivery of lipophilic drugs from o/w Pickering emulsions. Int J Pharm. 2009;371(1-2):56–63.
Shah PP, Desai RP, Channer D, Singh M. Enhanced skin permeation using polyarginine modified nanostructured lipid carriers. J Control Release. 2012;161(3):735–45.
Hussain A, Samad A, Singh SK, Ahsan MN, Haque MW, Faruk A, et al. Nanoemulsion gel-based topical delivery of an antifungal drug: in vitro activity and in vivo evaluation. Drug Deliv. 2016;23(2):642–7.
Kong M, Chen XG, Kweon DK, Park HJ. Investigations on skin permeation of hyaluronic acid based nanoemulsion as transdermal carrier. Carbohydr Polym. 2011;86(2):837–43.
Zheng Y, Ouyang WQ, Wei YP, Syed SF, Hao CS, Wang BZ, et al. Effects of Carbopol® 934 proportion on nanoemulsion gel for topical and transdermal drug delivery: A skin permeation study. Int J Nanomedicine. 2016;11:5971–87.
Changez M, Varshney M, Chander J, Dinda AK. Effect of the composition of lecithin/n-propanol/isopropyl myristate/water microemulsions on barrier properties of mice skin for transdermal permeation of tetracainehydrochloride: in vitro. Colloids Surf B: Biointerfaces. 2006;50(1):18–25.
Shakeel F, Baboota S, Ahuja A, Ali J, Shafiq S. Skin permeation mechanism and bioavailability enhancement of celecoxib from transdermally applied nanoemulsion. J Nanobiotechnol. 2008;6(1):1–11.
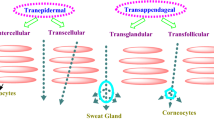
Fuchs E. Beauty is skin deep: the fascinating biology of the epidermis and its appendages. Harvey Lect. 1998;94:47–77.
Funding
This work was supported by the Science and Technology Project of Guangdong Province (grant numbers 201802010047); Innovation and Entrepreneurship Team special Fund project of Guangdong Province (grant numbers 2014ZT05Y018), and Biomedical Innovation Institution of Hong Kong & Guangdong Pharmaceutical University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Animal experiments were performed according to internationally accepted ethical guidelines for the care of laboratory animals, and were approved by the Institutional Animal Care and Use Committee of Guangdong Pharmaceutical University.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
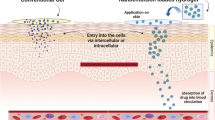
We report a nanoemulgel system with good penetrating ability and a sustained release effect that can reduces adverse reactions and side effects caused by clinical medication overdose. Here, we examine the preparation, characterization, permeability, release kinetics, rheology, and topical mechanism of a nanoemulgel.
Rights and permissions
About this article
Cite this article
Ma, Q., Zhang, J., Lu, B. et al. Nanoemulgel for Improved Topical Delivery of Desonide: Formulation Design and Characterization. AAPS PharmSciTech 22, 163 (2021). https://doi.org/10.1208/s12249-021-02035-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02035-5