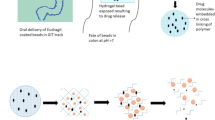
Abstract
The purpose of this study was to investigate the potential of Bletilla striata polysaccharide (BSP, a natural glucomannan material) for the development of a gastroretentive drug delivery system for the first time. Novel BSP-based porous wafer was prepared for levofloxacin hydrochloride (LFH) delivery by combining floating, swelling, and mucoadhesion mechanisms. The influences of BSP and ethyl cellulose (EC) on drug release and mucoadhesive strength were studied by 32 factorial design. The optimized matrix was coated with polycaprolactone (PCL) electrospun membrane by electrospinning and heat treatment technology. The optimized formula (F6, coated) exhibited Q4 h of 41.20 ± 1.90%, Q8 h of 76.49 ± 1.69%, and mucoadhesive strength of 86.11 ± 1.33 gf, and its drug release profile most closely resembled the Korsmeyer–Peppas model with anomalous diffusion driving mechanism. F6 (coated) also presented excellent buoyancy, preferred swelling characteristic due to the porous structure formed by freeze-drying. Meanwhile, the internal morphology, physical state, drug–excipient compatibility, and thermal behavior were recorded. The negligible cytotoxicity of F6 (coated) was observed in human gastric epithelial cell cultures. In the in vitro antimicrobial experiment, the prepared wafer exhibited obvious bacterial inhibition zone, and due to its longer gastric retention, the wafer also performed a more effective Helicobacter pylori clearance than free LFH in vivo.

Graphical abstract










Similar content being viewed by others
Abbreviations
- BSP:
-
Bletilla striata polysaccharide
- DSC:
-
Differential scanning calorimeter
- EC:
-
Ethyl cellulose
- FTIR:
-
Fourier transform infrared spectroscopy
- GES-1:
-
Human gastric mucosal epithelial cells
- GRDDS:
-
Gastroretentive drug delivery system
- H. pylori :
-
Helicobacter pylori
- LFH:
-
Levofloxacin hydrochloride
- PCL:
-
Polycaprolactone
- Q 4 h :
-
Cumulative release of the drug at 4 h
- Q 8 h :
-
Cumulative release of the drug at 8 h
References
El-Zahaby SA, Kassem AA, El-Kamel AH. Design and evaluation of gastroretentive levofloxacin floating mini-tablets-in-capsule system for eradication of Helicobacter pylori. Saudi Pharmaceutical Journal. 2014;22(6):570–9.
Antos D, Schneider-Brachert W, Bästlein E, Hänel C, Haferland C, Buchner M, et al. 7-Day triple therapy of Helicobacter pylori infection with levofloxacin, amoxicillin, and high-dose esomeprazole in patients with known antimicrobial sensitivity. Helicobacter. 2006;11(1):39–45.
Bera H, Maiti S, Saha S, Nayak AK. Biopolymers-based gastroretentive buoyant systems for therapeutic management of Helicobacter pylori infection. Polysaccharide Carriers for Drug Delivery; 2019. 713–736 p.
Lopes CM, Bettencourt C, Rossi A, Buttini F, Barata P. Overview on gastroretentive drug delivery systems for improving drug bioavailability. Int J Pharm. 2016;510(1):144–58.
Hwang KM, Cho CH, Tung NT, Kim JY, Rhee YS, Park ES. Release kinetics of highly porous floating tablets containing cilostazol. Eur J Pharm Biopharm. 2017;115(2):39–51.
Tadros MI. Controlled-release effervescent floating matrix tablets of ciprofloxacin hydrochloride: development, optimization and in vitro-in vivo evaluation in healthy human volunteers. Eur J Pharm Biopharm. 2010;74(2):332–9.
Rajput P, Singh D, Pathak K. Bifunctional capsular dosage form: novel fanicular cylindrical gastroretentive system of clarithromycin and immediate release granules of ranitidine HCl for simultaneous delivery. Int J Pharm. 2014;461(1–2):310–21.
Patel A, Modasiya M, Shah D, Patel V. Development and in vivo floating behavior of verapamil HCl intragastric floating tablets. AAPS PharmSciTech. 2009;10(1):310–5.
Bhalla S, Nagpal M. Comparison of various generations of superporous hydrogels based on chitosan-acrylamide and in vitro drug release. ISRN Pharmaceutics. 2013;2013:1–8.
Su C-Y, Ho H-O, Chen Y-C, Yu Y-T, Liu D-Z, Chao F-C, et al. Complex hydrogels composed of chitosan with ring-opened polyvinyl pyrrolidone as a gastroretentive drug dosage form to enhance the bioavailability of bisphosphonates. Sci Rep. 2018;8(1):1–12.
Ruiz-Caro R, Veiga MD. In vitro evaluation of acyclovir/chitosan floating systems. Materials. 2010;3(12):5195–211.
Kim JY, Kim SH, Rhee YS, Park CW, Park ES. Preparation of hydroxypropylmethyl cellulose-based porous matrix for gastroretentive delivery of gabapentin using the freeze-drying method. Cellulose. 2013;20(6):3143–54.
Mandal UK, Chatterjee B, Senjoti FG. Gastro-retentive drug delivery systems and their in vivo success: a recent update. Int J Pharm. 2016.
Auriemma G, Cerciello A, Sansone F, Morello S, Aquino RP. Polysaccharides based gastroretentive system to sustain piroxicam release: development and in vivo prolonged anti-inflammatory effect. Int J Biol Macromol. 2018.
Yao J. Tiao He Yi Wei granule, a traditional Chinese medicine, against ethanol-induced gastric ulcer in mice. Evid Based Complement Alternat Med. 2015;2015:1–8.
Qu Y, Li C, Zhang C, Zeng R, Fu C. Optimization of infrared-assisted extraction of Bletilla striata polysaccharides based on response surface methodology and their antioxidant activities. Carbohydr Polym. 2016;148:345–53.
Liao Z, Zeng R, Hu L, Maffucci KG, Qu Y. Polysaccharides from tubers of Bletilla striata: physicochemical characterization, formulation of buccoadhesive wafers and preliminary study on treating oral ulcer. Int J Biol Macromol. 2019;122:1035–45.
Hu L, Liao Z, Hu Q, Maffucci KG, Qu Y. Novel Bletilla striata polysaccharide microneedles: fabrication, characterization, and in vitro transcutaneous drug delivery. Int J Biol Macromol. 2018;117:928–36.
Cui X, Zhang X, Yang Y, Wang C, Zhang C, Peng G. Preparation and evaluation of novel hydrogel based on polysaccharide isolated from Bletilla striata. Pharm Dev Technol. 2017;22(8):1001–11.
Kim JY, Seo JW, Rhee YS, Park CW, Park ES. Freeze-dried highly porous matrix as a new gastroretentive dosage form for ecabet sodium: in vitro and in vivo characterizations. J Pharm Sci. 2014;103(1):262–73.
Said Z, Baker SR, Hansen J, D’Apice K, Thornhill MH, Madsen LS, et al. Pre-clinical evaluation of novel mucoadhesive bilayer patches for local delivery of clobetasol-17-propionate to the oral mucosa. Biomaterials. 2018;178:134–46.
Lee WL, Tan JWM, Tan CN, Loo SCJ. Modulating drug release from gastric-floating microcapsules through spray-coating layers. PLoS One. 2014;9(12):1–16.
Abriata JP, Turatti RC, Luiz MT, Raspantini GL, Tofani LB, do Amaral RLF, et al. Development, characterization and biological in vitro assays of paclitaxel-loaded PCL polymeric nanoparticles. Materials Science and Engineering C. 2019;96:347–55.
Qi X, Chen H, Rui Y, Yang F, Ma N, Wu Z. Floating tablets for controlled release of ofloxacin via compression coating of hydroxypropyl cellulose combined with effervescent agent. Int J Pharm. 2015;489(1–2):210–7.
Zhao Q, Gao B, Ma L, Lian J, Deng L, Chen J. Innovative intragastric ascaridole floating tablets: development, optimization, and in vitro-in vivo evaluation. Int J Pharm. 2015;496(2):432–9.
College JSS, Nagar SS. Development of a gastroretentive drug delivery system based on superporous hydrogel. N Vishal Gupta * and HG Shivakumar. 2010;9(6):257–64.
Sharma OP, Shah MV, Parikh DC, Mehta TA. Formulation optimization of gastroretentive drug delivery system for allopurinol using experimental design. Expert Opinion on Drug Delivery. 2014;12(4):513–24.
El-Zahaby SA, Kassem AA, El-Kamel AH. Formulation and in vitro evaluation of size expanding gastro-retentive systems of levofloxacin hemihydrate. Int J Pharm. 2014;464(1–2):10–8.
Canal C, Aparicio RM, Esquena J, Girona J, Group TE. Drug delivery properties of macroporous polystyrene solid foams. J Pharm Pharm Sci. 2012;15(1):197–207.
Hao S, Wang Y, Wang B. Sinking-magnetic microparticles prepared by the electrospray method for enhanced gastric antimicrobial delivery. Mol Pharm. 2014;11(5):1640–50.
Hao S, Wang Y, Wang B, Zou Q, Zeng H, Chen X, et al. A novel gastroretentive porous microparticle for anti-Helicobacter pylori therapy: preparation, in vitro and in vivo evaluation. Int J Pharm. 2014;463(1):10–21.
Siafaka PI, Zisi AP, Exindari MK, Karantas ID, Bikiaris DN. Porous dressings of modified chitosan with poly(2-hydroxyethyl acrylate) for topical wound delivery of levofloxacin. Carbohydr Polym. 2016;143:90–9.
Aoki H, Iwao Y, Mizoguchi M, Noguchi S, Itai S. Clarithromycin highly-loaded gastro-floating fine granules prepared by high-shear melt granulation can enhance the efficacy of Helicobacter pylori eradication. Eur J Pharm Biopharm. 2015;2:2–7.
Zhang X, Zhang Y, Han H. Formulation optimization of gastro-retention tablets of paeonol and efficacy in treatment of experimental gastric ulcer. Chem Pharm Bull. 2017;1200.
Gambhire MN, Ambade KW, Kurmi SD, Kadam VJ, Jadhav KR. Development and in vitro evaluation of an oral floating matrix tablet formulation of diltiazem hydrochloride. AAPS PharmSciTech. 2007;8(3):E166–74.
Priyadarshini R, Nandi G, Changder A, Chowdhury S, Chakraborty S, Ghosh LK. Gastroretentive extended release of metformin from methacrylamide-g-gellan and tamarind seed gum composite matrix. Carbohydrate Polymers. 2015.
Ahmed A, Getti G, Boateng J. Ciprofloxacin-loaded calcium alginate wafers prepared by freeze-drying technique for potential healing of chronic diabetic foot ulcers. Drug Delivery and Translational Research. 2018;8(6):1751–68.
Acharya S, Patra S, Pani NR. Optimization of HPMC and carbopol concentrations in non-effervescent floating tablet through factorial design. Carbohydrate Polymers. 2014;102(1).
Hauptstein S, Müller C, Dünnhaupt S, Laffleur F, Bernkop-schnürch A. Preactivated thiomers: evaluation of gastroretentive minitablets. Int J Pharm. 2013:1–7.
Qin C, Wu M, Xu S, Wang X, Shi W, Dong Y, et al. Design and optimization of gastro-floating sustained-release tablet of pregabalin: in vitro and in vivo evaluation. International Journal of Pharmaceutics. 2018;545(1–2).
Bera H, Gaini C, Kumar S, Sarkar S, Boddupalli S, Ippagunta SR. HPMC-based gastroretentive dual working matrices coated with Ca+ 2ion crosslinked alginate-fenugreek gum gel membrane. Mater Sci Eng C. 2016;67:170–81.
Bera H, Ippagunta SR, Kumar S, Vangala P. Core-shell alginate-ghatti gum modified montmorillonite composite matrices for stomach-specific flurbiprofen delivery. Mater Sci Eng C. 2017;76:715–26.
Jalvandi J, White M, Gao Y, Truong YB, Padhye R, Kyratzis IL. Polyvinyl alcohol composite nanofibres containing conjugated levofloxacin-chitosan for controlled drug release. Mater Sci Eng C. 2017;73:440–6.
Islan GA, Cacicedo ML, Bosio VE, Castro GR. Development and characterization of new enzymatic modified hybrid calcium carbonate microparticles to obtain nano-architectured surfaces for enhanced drug loading. J Colloid Interface Sci. 2015;439:76–87.
Imam SS, Nasir S, Bukhari A, Ahmed J, Ali A. Formulation and optimization of levofloxacin loaded chitosan nanoparticle for ocular delivery: in-vitro characterization, ocular tolerance and antibacterial activity. International Journal of Biological Macromolecules. 2017.
Chen Z, Cheng L, He Y, Wei X. Extraction, characterization, utilization as wound dressing and drug delivery of Bletilla striata polysaccharide: a review. Int J Biol Macromol. 2018.
Zhang C, Gao F, Gan S, He Y, Chen Z, Liu X, et al. Chemical characterization and gastroprotective effect of an isolated polysaccharide fraction from Bletilla striata against ethanol-induced acute gastric ulcer. Food Chem Toxicol. 2019;131(5):110539.
Islan GA, Dini C, Bartel LC, Bolzán AD, Castro GR. Characterization of smart auto-degradative hydrogel matrix containing alginate lyase to enhance levofloxacin delivery against bacterial biofilms. Int J Pharm. 2015;496(2):953–64.
Verma A, Dubey J, Hegde RR, Rastogi V, Pandit JK. Helicobacter pylori: past, current and future treatment strategies with gastroretentive drug delivery systems. J Drug Target. 2016;24(10):897–915.
Huanbutta K, Sangnim T. Design and development of zero-order drug release gastroretentive floating tablets fabricated by 3D printing technology. Journal of Drug Delivery Science and Technology. 2019;52(5):831–7.
Funding
This research was supported by the National Natural Science Foundation of China (81603309), the National Key Research and Development Program of China (2017YFC1700705), the Sichuan Provincial Scholars for Science and Technology Activities for Overseas Researchers (2018-68), and the Key Projects of Central Universities (2018NZD18).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
All the animal experiments were approved and supervised/conducted by the Chengdu University of Traditional Chinese Medicine Ethics Committee of Care and Use of Laboratory Animals, and the animals were house and handled according to the University Unit for Laboratory Animal Medicine guidelines.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 74 kb)
Rights and permissions
About this article
Cite this article
Li, Z., Zeng, R., Yang, L. et al. Development and Characterization of PCL Electrospun Membrane-Coated Bletilla striata Polysaccharide-Based Gastroretentive Drug Delivery System. AAPS PharmSciTech 21, 66 (2020). https://doi.org/10.1208/s12249-019-1607-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1607-5