Abstract
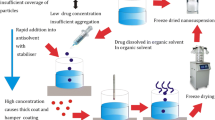
Kaempferia parviflora (KP) is an herbal medicine for enhancement of physical fitness and male sexual function improvement with low oral absorption of the main active compounds, methoxyflavones. The purpose of this study is to optimize the preparation of nanosuspensions of KP extract for enhancing intestinal absorption using antisolvent precipitation technique which is an accessible nanomanufacturing methodology in the small industrial factory. Nanosuspensions were prepared using various types and concentrations of stabilizers. Then, the dry powder of KP nanosuspension was produced by spray drying. Its dissolution rate was determined using USP dissolution apparatus II. The rat everted intestinal sac was tested to confirm the improvement of intestinal absorption of KP nanosuspension. The result showed that 3% sodium lauryl sulfate (SLS) was the optimal condition for covering the nano-size of KP nanosuspension. KP nanosuspensions had particle sizes ranging from 100 to 300 nm with narrow size distribution (PDI < 0.60) and zeta potential at − 58 to − 70 mV. These characteristics were stable at 4°C and 25°C/60%RH for 1-month storage. Its methoxyflavones content also unchanged at 4°C and 25°C/60%RH for 1-month storage. KP nanosuspension released > 80% of the methoxyflavones within 30 min both in 0.1 N HCl and 0.01 M phosphate buffer solution (pH 6.8). Moreover, the developed nanosuspension dramatically improved the rat intestinal absorption about 10-fold. Therefore, the KP nanosuspension was successfully prepared. It has relatively high stability, fast dissolution rate, and high intestinal absorption.




Similar content being viewed by others
References
Gunasekaran T, Haile T, Nigusse T, Dhanaraju MD. Nanotechnology: an effective tool for enhancing bioavailability and bioactivity of phytomedicine. Asian Pac J Trop Biomed. 2014:4, S1–S7. https://doi.org/10.12980/apjtb.4.2014c980.
Green MR, Manikhas GM, Orlov S, Afanasyev B, Makhson AM, Bhar P, et al. Abraxane®, a novel Cremophor®-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Ann Oncol. 2006;17:1263–8. https://doi.org/10.1093/annonc/mdl104.
Malakar J, Basu A, Ghosh A. Nanosuspension: A Nano-heterogeneous carrier for drug delivery system. Int J Pharm Biol Arch. 2012;3:4–13.
Shetea G, Jaina H, Punja D, Prajapata H, Akotiyaa P, Bansala AK. Stabilizers used in nano-crystal based drug delivery systems. J Excipients Food Chem. 2014;5:184–209.
Thipparaboina R, Chavan R, Nalini S. Nanocrystals for delivery of therapeutic agents. In: Jana S, Jana S, editors. Part Technol Deliv Ther. Singapore: Springer; 2017. p. 291–316.
Yadav D, Kumar N. Nanonization of curcumin by antisolvent precipitation: Process development, characterization, freeze drying and stability performance. Int J Pharm. 2014;477:564–77. https://doi.org/10.1016/j.ijpharm.2014.10.070.
Patel VR, Agrawal YK. Nanosuspension: an approach to enhance solubility of drugs. J Adv Pharm Technol Res. 2011;2:81–7. https://doi.org/10.4103/2231-4040.82950.
Shi-Ying J, Jin H, Shi-Xiao J, Qing-Yuan LV, Jin-Xia B, Chen HG, et al. Characterization and evaluation in vivo of baicalin-nanocrystals prepared by an ultrasonic-homogenization-fluid bed drying method. Chin J Nat Med. 2014;12:71–80. https://doi.org/10.1016/S1875-5364(14)60012-1.
Mauludin R, Müller RH, Keck CM. Development of an oral rutin nanocrystal formulation. Int J Pharm. 2009;370:202–9. https://doi.org/10.1016/j.ijpharm.200811.029.
Wang Y, Zhang D, Liu Z, Liu G, Duan C, Jia L, et al. In vitro and in vivo evaluation of silybin nanosuspensions for oral and intravenous delivery. Nanotechnology. 2010;21:155104. https://doi.org/10.1088/0957-4484/21/15/155104.
Mitri K, Shegokar R, Gohla S, Anselmi C, Müller RH. Lutein nanocrystals as antioxidant formulation for oral and dermal delivery. Int J Pharm. 2011;420:141–6. https://doi.org/10.1016/j.ijpharm.2011.08.026.
Wang Y, Ma Y, Ma Y, Du Y, Liu Z, Zhang D, et al. Formulation and pharmacokinetics evaluation of puerarin nanocrystals for intravenous delivery. J Nanosci Nanotechnol. 2012;12:6176–84. https://doi.org/10.1166/jnn.2012.6436.
Sadeghi F, Ashofteh M, Homayouni A, Abbaspour M, Nokhodchi A, Garekani HA. Antisolvent precipitation technique: A very promising approach to crystallize curcumin in presence of polyvinyl pyrrolidon for solubility and dissolution enhancement. Colloids Surf B: Biointerfaces. 2016;147:258–64. https://doi.org/10.1016/j.colsurfb.2016.08.004.
Bi C, Miao XQ, Chow SF, Wu WJ, Yan R, Liao YH, et al. Particle size effect of curcumin nanosuspensions on cytotoxicity, cellular internalization, in vivo pharmacokinetics and biodistribution. Nanomedicine. 2017;13:943–53. https://doi.org/10.1016/j.nano.2016.11.004.
Wu W, Zu Y, Wang L, Wang L, Wang H, Li Y, et al. Preparation, characterization and antitumor activity evaluation of apigenin nanoparticles by the liquid antisolvent precipitation technique. Drug Deliv. 2017;24:1713–20. https://doi.org/10.1080/10717544.2017.1399302.
Hao J, Gao Y, Zhao J, Zhang J, Li Q, Zhao Z, et al. Preparation and Optimization of Resveratrol Nanosuspensions by Antisolvent Precipitation Using Box-Behnken Design. AAPS PharmSciTech. 2015;16:118–28. https://doi.org/10.1208/s12249-014-0211-y.
Kummee S, Tewtrakul S, Subhadhirasakul S. Antimicrobial activity of the ethanol extract and compounds from the rhizomes of Kaempferia parviflora. Songklanakarin J Sci Technol. 2008;30:463–6.
Tewtrakul S, Subhadhirasakul S, Kummee S. Anti-allergic activity of compounds from Kaempferia parviflora. J Ethnopharmacol. 2008;116:191–3. https://doi.org/10.1016/j.jep.2007.10.042.
Banjerdpongchai R, Suwannachot K, Rattanapanone V, Sripanidkulchai B. Ethanolic rhizome extract from Kaempferia parviflora Wall. ex. Baker induces apoptosis in HL-60 cells. Asian Pac J Cancer Prev. 2008;9:595–600.
Leardkamolkarn S, Tiamyuyen V, Sripanidkulchai B. Pharmacological activity of Kaempferia parviflora extract against human bile duct cancer cell lines. Asian Pac J Cancer Prev. 2009;10:695–8.
Azuma T, Kayano SI, Matsumura Y, Konishi Y, Tanaka Y, Kikuzaki H. Antimutagenic and α-glucosidase inhibitory effects of constituents from Kaempferia parviflora. Food Chem. 2011;125. https://doi.org/10.1016/j.foodchem.2010.09.033.
Thao NP, Luyen BTT, Lee SH, Jang HD, Kim YH. Anti-osteoporotic and antioxidant activities by rhizomes of Kaempferia parviflora wall. Ex Baker. Nat Prod Sci. 2016;22:13–9. https://doi.org/10.20307/nps.2016.22.1.13.
Malakul W, Ingkaninan K, Sawasdee P, Woodman OL. The ethanolic extract of Kaempferia parviflora reduces ischaemic injury in rat isolated hearts. J Ethnopharmacol. 2011;137:184–91. https://doi.org/10.1016/.j.jep.2011.05.004.
Rujjanawate C, Kanjanapothi D, Amornlerdpison D, Pojanagaroon S. Anti-gastric ulcer effect of Kaempferia parviflora. J Ethnopharmacol. 2005;102:120–2. https://doi.org/10.1016/j.jep.2005.03.035.
Sae-wong C, Tansakul P, Tewtrakul S. Anti-inflammatory mechanism of Kaempferia parviflora in murine macrophage cells (RAW 264.7) and in experimental animals. J Ethnopharmacol. 2009;124:576–80. https://doi.org/10.1016/j.jep.2009.04.059.
Hawiset T, Muchimapura S, Wattanathorn J, Sripanidkulchai B. Screening neuropharmacological activities of Kaempferia parviflora (krachai dam) in healthy adult male rats. Am J Appl Sci. 2011;8:695–702. https://doi.org/10.3844/ajassp.2011.695.702.
Wattanathorn J, Pangpookiew P, Sripanidkulchai K, Muchimapura S, Sripanidkuchai B. Evaluation of the anxiolytic and antidepressant effects of alcoholic extract of Kaempferia parviflora in aged rats. Am J Agric Biol Sci. 2007;2:94–8. https://doi.org/10.3844/ajabssp2007.94.98.
Welbat JU, Chaisawang P, Chaijaroonkhanarak W, Prachaney P, Pannangrong W, Sripanidkulchai B, et al. Kaempferia parviflora extract ameliorates the cognitive impairments and the reduction in cell proliferation induced by valproic acid treatment in rats. Ann Anat. 2016;206:7–13. https://doi.org/10.1016/j.aanat.2016.04.029.
Akase T, Shimada T, Terabayashi S, Ikeya Y, Sanada H, Aburada M. Antiobesity effects of Kaempferia parviflora in spontaneously obese type II diabetic mice. J Nat Med. 2011;65:73–80. https://doi.org/10.1007/s11418-010-0461-2.
Matsushita M, Yoneshiro T, Aita S, Kamiya T, Kusaba N, Yamaguchi K, et al. Kaempferia parviflora extract increases whole-body energy expenditure in humans: roles of brown adipose tissue. J Nutr Sci Vitaminol Nutr Sci Vitaminol. 2015;61:79–83. https://doi.org/10.3177/jnsv.61.79.
Kobayashi H, Horiguchi-Babamoto E, Suzuki M, Makihara H, Tomozawa H, Tsubata M, et al. Effects of ethyl acetate extract of Kaempferia parviflora on brown adipose tissue. J Nat Med. 2016;70:54–61. https://doi.org/10.1007/s11418-015-0936-2.
Wannanon P, Wattanathorn J, Tong-Un T, Pangphukiew P, Muchimapura S, Sripanidkulchai B, et al. Efficacy assessment of Kaempferia Parviflora for the management of Erectile Dysfunction. Online J Biol Sci. 2012;12:149–55. https://doi.org/10.3844/ojbsci.2012.149.155.
Wattanathorn J, Muchimapura S, Tong-Un T, Saenghong N, Thukhum-Mee W, Sripanidkulchai B. Positive modulation effect of 8-week consumption of Kaempferia parviflora on health-related physical fitness and oxidative status in healthy elderly volunteers. Evid Based Complement Altern Med. 2012;732816. https://doi.org/10.1155/2012/732816.
Promthep K, Eungpinichpong W, Sripanidkulchai B, Chatchawan U. Effect of Kaempferia parviflora extract on physical fitness of soccer players: a randomized double-blind placebo-controlled trial. Med Sci Monit Basic Res. 2015;21:100–8. https://doi.org/10.12659/msmbr.894301.
Chivapat S, Chavalittumrog P, Phadungpat S, Pama KK, Chansuvanich N, Attawish A. Acute and chronic toxicity study of Kaempferia parviflora wall ex. baker powder. J Thai Tradit Altern Med. 2004;2:3–16.
Sutthanut K, Sripanidkulchai B, Yenjai C, Jay M. Simultaneous identification and quantitation of 11 flavonoid constituents in Kaempferia parviflora by gas chromatography. J Chromatogr A. 2007;1143:227–33. https://doi.org/10.1016/j.chroma.2007.01.033.
Mekjaruskul C, Jay M, Sripanidkulchai B. Pharmacokinetics, bioavailability, tissue distribution, excretion, and metabolite identification of methoxyflavones in Kaempferia parviflora extract in rats. Drug Metab Dispos. 2012;40. https://doi.org/10.1124/dmd/112.047142.
Mekjaruskul C, Yang Y-T, Leed MGD, Sadgrove MP, Jay M, Sripanidkulchai B. Novel formulation strategies for enhancing oral delivery of methoxyflavones in Kaempferia parviflora by SMEDDS or complexation with 2-hydroxypropyl-β- cyclodextrin. Int J Pharm. 2013;445. https://doi.org/10.1016/j.ijpharm.2013.01.052.
Cheng G, Hu R, Ye L, Wang B, Gui Y, Gao S, et al. Preparation and in vitro/in vivo evaluation of puerarin solid self-microemulsifying drug delivery system by spherical crystallization technique. AAPS PharmSciTech. 2016;17:1336–46. https://doi.org/10.1208/s12249-015-0469-8.
Kaur J, Bawa P, Rajesh SY, Sharma P, Ghai D, Jyoti J, et al. Formulation of curcumin nanosuspension using box-behnken design and study of impact of drying techniques on its powder characteristics. Asian J Pharm Clin Res. 2017;(September):43–51. https://doi.org/10.22159/ajpcr.2017.v10s4.21335.
Zu Y, Wu W, Zhao X, Li Y, Wang W, Zhong C, et al. Enhancement of solubility, antioxidant ability and bioavailability of taxifolin nanoparticles by liquid antisolvent precipitation technique. Int J Pharm. 2014;471:366–76. https://doi.org/10.1016/j.ijpharm.2014.05.049.
Pawar RN, Chavan SNMM. Development, characterization and evaluation of tinidazole nanosuspension for treatment of amoebiasis. J Nanomed Nanotechnol. 2017;7:1–4. https://doi.org/10.4172/2157-7439.1000413.
Dzakwan M, Pramukantoro GE, Mauludin R, Wikarsa S. Formulation and characterization of fisetin nanosuspension. IOP Conf Ser Mater Sci Eng. 2017:1–5. https://doi.org/10.1088/1757-899X/259/1/012016.
Shelar DB, Pawar SK, Vavia PR. Fabrication of isradipine nanosuspension by anti-solvent microprecipitation-high-pressure homogenization method for enhancing dissolution rate and oral bioavailability. Drug Deliv Transl Res. 2013;3:384–91. https://doi.org/10.1007/s13346-012-0081-3.
Pouretedal HR. Preparation and characterization of azithromycin nanodrug using solvent/antisolvent method. Int Nano Lett. 2014;4:103–9. https://doi.org/10.1007/s40089-014-0103-x.
Shariare MH, Sharmin S, Jahan I, Reza HM, Mohsin K. The impact of process parameters on carrier free paracetamol nanosuspension prepared using different stabilizers by antisolvent precipitation method. J Drug Deliv Sci Technol. 2018;43:122–8. https://doi.org/10.1016/j.ddst.2017.10.001.
Dolenc A, Kristl J, Baumgartner S, Planinšek O. Advantages of celecoxib nanosuspension formulation and transformation into tablets. Int J Pharm. 2009;376:204–12. https://doi.org/10.1016/j.ijpharm.2009.04.038.
Saokham P, Muankaew C, Jansook P, Loftsson T. Solubility of cyclodextrins and drug/cyclodextrin complexes. Molecules. 2018;23:1–15. https://doi.org/10.3390/molecules23051161.
Rowe RC, Sheskey PJ, Owen SC. Handbook of pharmaceutical excipients. 5th ed. American Pharmacists Association: Washington, D.C; 2006.
Acknowledgments
The Center for research and development of herbal health products, Khon Kaen University, Thailand, and Faculty of Pharmacy, Mahasarakham University, Thailand, were acknowledged for facility support. We would like to thank Dr. Adrian Roderick Plant for his assistance proofreading this article.
Funding
This study was financially supported by the Agricultural Research Development Agency (Public Organization) (Grant No. CRP5705021730), the Office of the Higher Education Commission, Thailand, and the Thailand Research Fund (Grant No. MRG6080035).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mekjaruskul, C., Sripanidkulchai, B. Kaempferia parviflora Nanosuspension Formulation for Scalability and Improvement of Dissolution Profiles and Intestinal Absorption. AAPS PharmSciTech 21, 52 (2020). https://doi.org/10.1208/s12249-019-1588-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1588-4